Glutamate dehydrogenase mutant and application thereof
A technique for glutamate dehydrogenase and mutants, which is applied in the field of glutamate dehydrogenase mutants and its applications, and can solve the problems of cumbersome process and low optical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: Construction of glutamate dehydrogenase mutant library
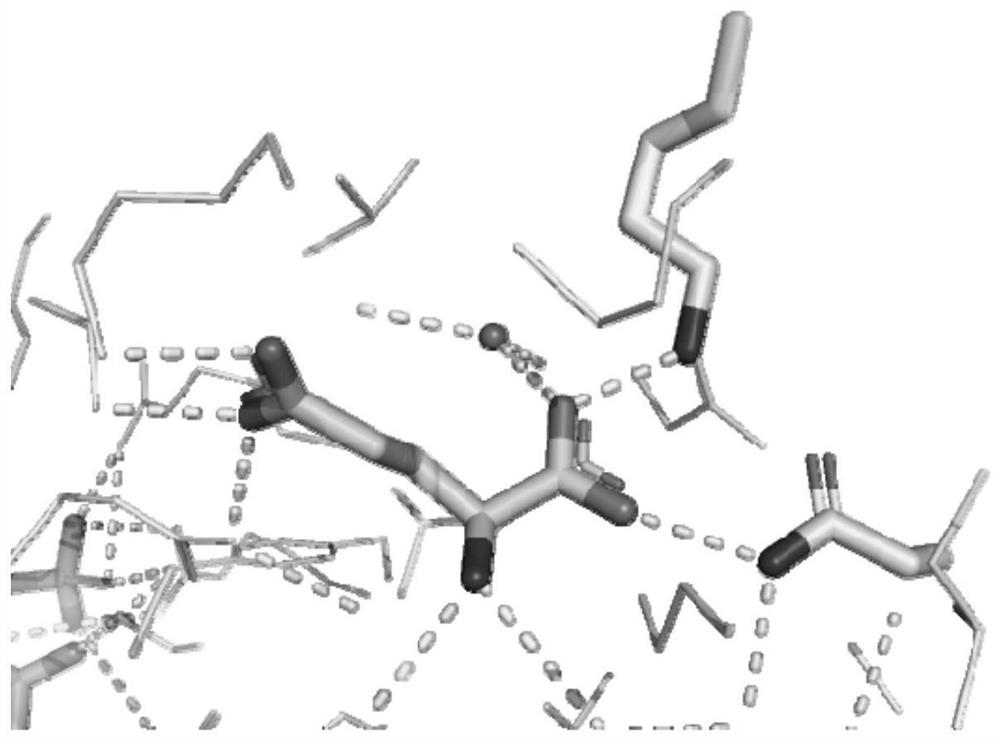
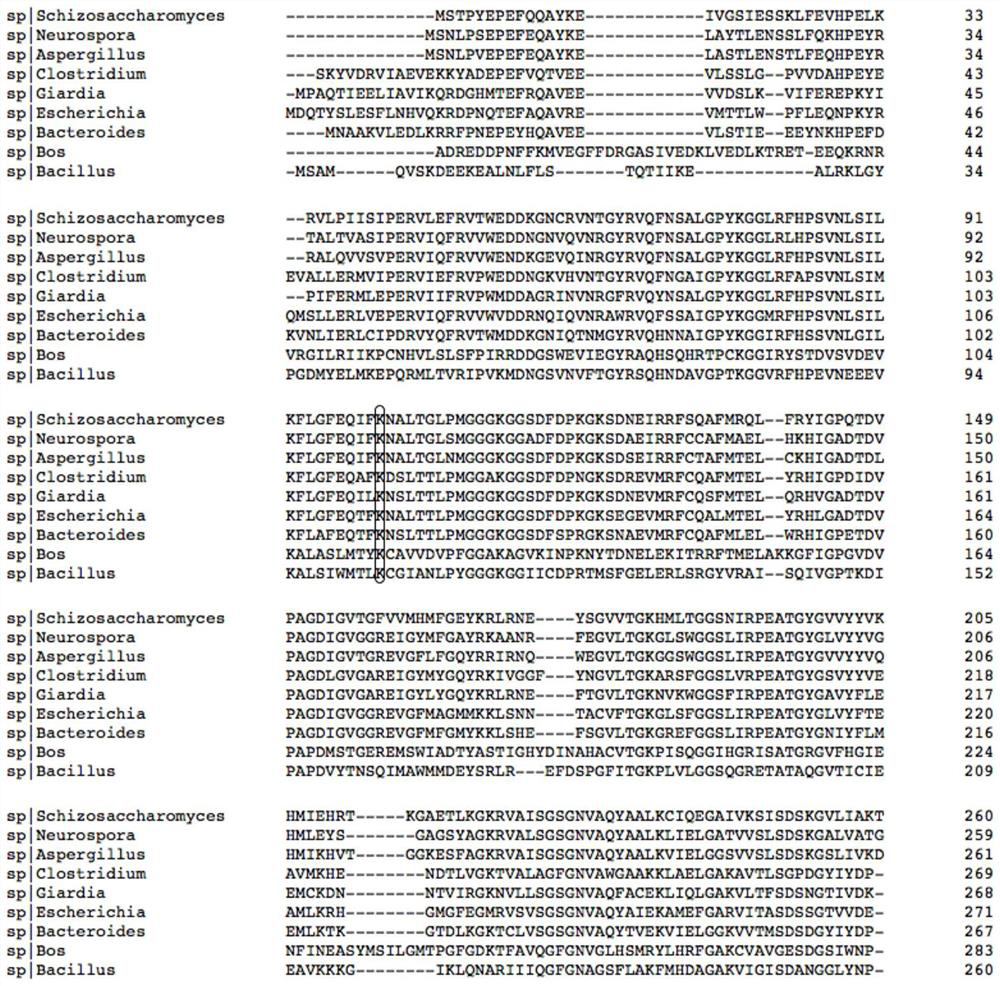
[0097] figure 1 is the crystal structure of glutamate dehydrogenase derived from bovine liver (PDB: 6dhd), which shows the interaction mode of the substrate glutamate and the enzyme; the amino acid residues 115K and 350N shown in the stick structure are related to the substrate The two oxygens of the main chain carboxyl group of glutamic acid interact to anchor the substrate main chain; it is found by sequence comparison (such as figure 2 Shown), these two amino acid residues are conserved sites in glutamate dehydrogenase, so the two sites are used as transformation objects.
[0098] The recombinant plasmid pET-28a-GluDH and the recombinant strain E.coli BL21(DE3) / pET-28a- GluDH, the recombinant strain E.coli BL21(DE3) / pET-28a-GluDH was named E.coli BL21(DE3) / pET-28a-WT1, and inoculated into LB solid medium for culture.
[0099] According to the target gene sequence upstream and downstream of the...
Embodiment 2
[0105] Embodiment 2: Primary screening of glutamate dehydrogenase mutant library
[0106] Specific steps are as follows:
[0107] (1) Pick the single colony of the recombinant bacterium BL21(DE3) / pET-28a-fusion protein obtained in Example 1, and transfer it to a 96-well deep-well plate (each well contains 300 μL LB liquid medium and 50 μg / mL kanamycin), and incubated at 37°C and 200rpm for 10-12h.
[0108] (2) Transfer 50 μL culture from each well obtained after incubation in step (1) to the second 96-deep well plate (each well contains 400 μL LB liquid medium and 50 μg / mL kanamycin in the corresponding wells in the prime), and a second 96-deep well plate was incubated at 37°C and 250rpm for 3-4h. Afterwards, 50 μL of LB liquid medium containing 2 mM IPTG was added to each well of the second 96 deep-well plate to induce protein expression, and then the second 96 deep-well plate was incubated at 20 °C and 250 rpm for 16- 18h.
[0109] (3) The product obtained in step (2) i...
Embodiment 3
[0112] Embodiment 3: the preparation of crude enzyme liquid of glutamic acid dehydrogenase
[0113] Specific steps are as follows:
[0114] (1) Strain activation: the E.coli BL21(DE3) / pET-28a-WT1 obtained in Example 1 and the genetically engineered bacteria containing the glutamate dehydrogenase mutant gene obtained in Example 2 were respectively: BL21(DE3) / pET-28a-K116S / N348L, BL21(DE3) / pET-28a-K116E / N348M, BL21(DE3) / pET-28a-K116Q / N348M were inoculated into test tubes containing 5 mL of LB liquid medium, Cultivate at 37° C. and 200 rpm for 6-8 hours to obtain seed liquid.
[0115] (2) Preparation of crude enzyme solution: respectively inoculate the activated seed solution into a conical flask containing 100mL LB liquid medium at an inoculum size of 2% (v / v), and cultivate for 2-3h at 37°C and 200rpm , to OD 600 After adding IPTG to a final concentration of 0.1mmol / L, continue to cultivate at 17°C and 200rpm for 12-17h to obtain a fermentation broth; respectively centrifuge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com