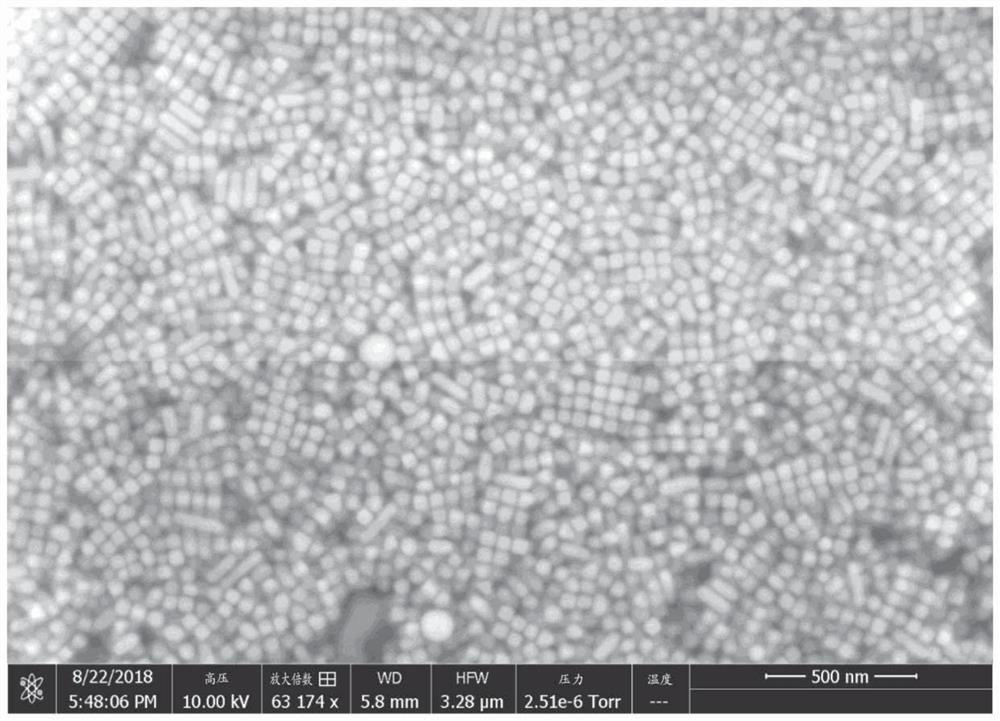
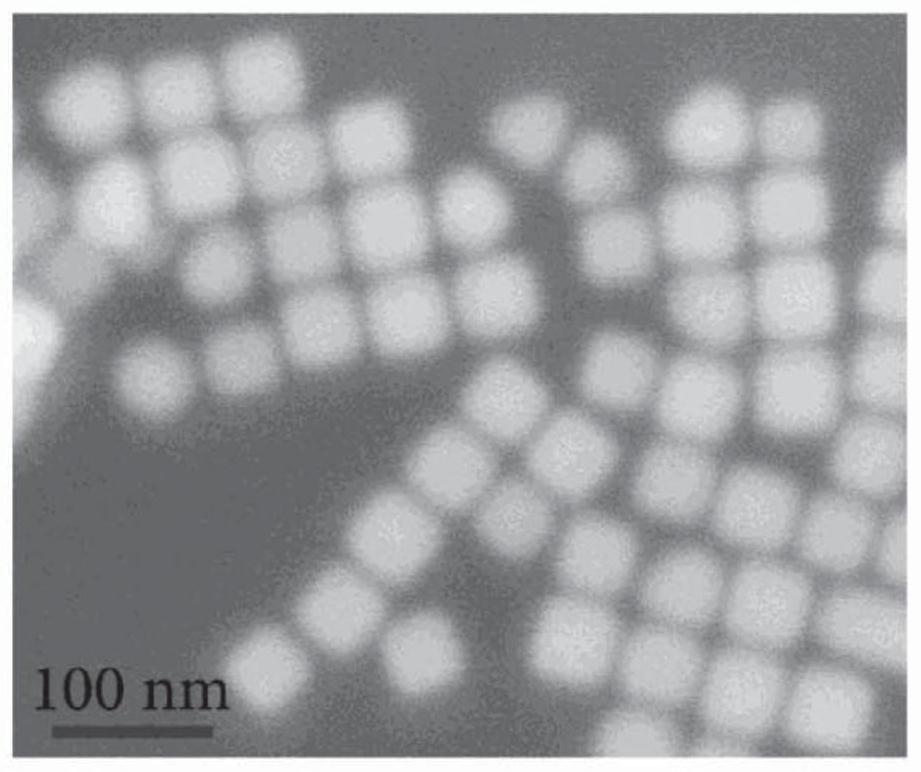
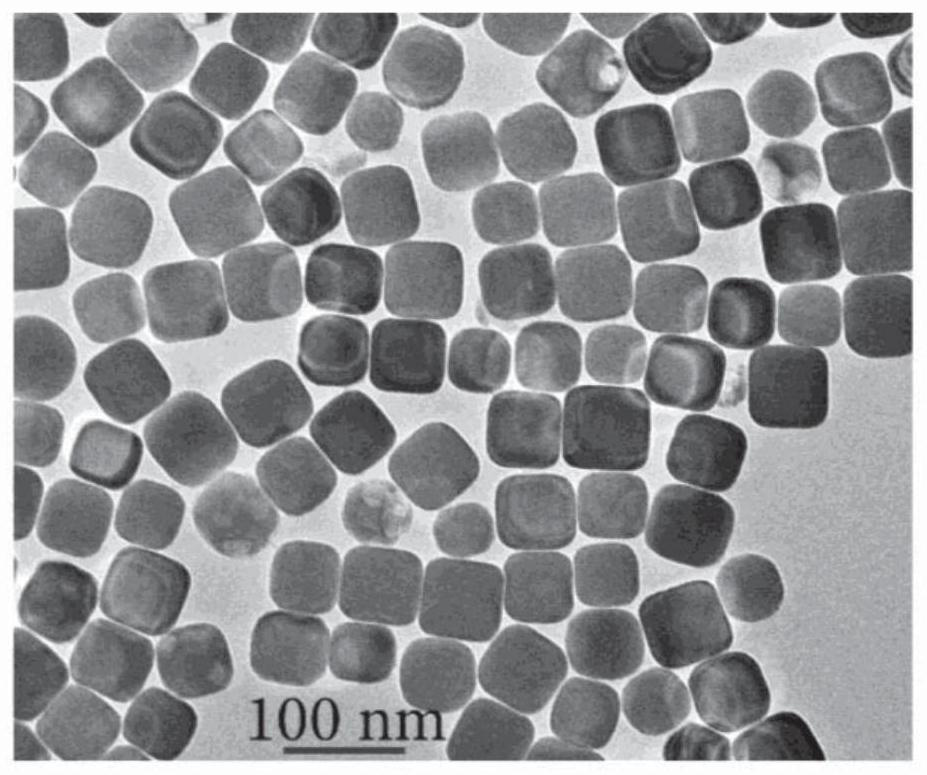
Method for preparation of copper nanocubes utilizing tributylphosphine as a ligand
A tributylphosphine, copper nanotechnology, applied in nanotechnology, chemical instruments and methods, metal processing equipment and other directions, can solve problems such as unclear formation mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0056] Embodiment 1: the preparation of Cu-TDA precursor complex solution
[0057] Copper chloride (99.0%), tributylphosphine (TBP, 99%), trioctylphosphine (TOP, 97%), oleylamine (OLA, 70%), toluene (99.9%), acetone (99%) and chloroform (99.9%) and 1-octadecene (ODE, 98%) were purchased from Sigma-Aldrich. Tetradecylamine (TDA, >96%) was purchased from Tokyo Chemical Industry Co., Ltd. (TCI). Hexane (99%), methanol (99%) and ethanol (200 proof) were purchased from Fisher Chemicals. All chemicals were used as received unless otherwise stated.
[0058] in Ar or N 2 100 mg of copper(I) chloride (1.0 mmol), 240 mg of TDA and 2 mL of ODE were added to the flask under flow. in Ar or N 2 After purging for 20 minutes, the mixed solution was heated to 200° C. and kept at this temperature for 10 minutes. The amount of copper(I) chloride was varied from 50 mg to 600 mg, while the amounts of TDA and TBP were increased from 120 mg to 1.44 g and from 0.5 mL to 6.0 mL, respectively. ...
Embodiment II
[0059] Example II: Synthesis of Cu nanocubes
[0060] 6.0 mL of oleylamine (OLA, 70%) was loaded into a 25 mL three-necked flask, where oxygen was removed by Ar purging for 20 min. 1.0 mL of tributylphosphine (TBP, 4.0 mmol) was injected into the flask under Ar flow. After 20 minutes of Ar flow, the flask was placed in a heating mantle with a temperature controller and rapidly heated to 300°C at a heating rate of 15-25°C / min. Next, 2 mL of Cu-TDA complex solution was quickly injected into the heated flask and the reaction solution turned red. The reaction was held at 300°C for 30 minutes. The reaction solution was then naturally cooled to room temperature, and injected with 5 mL of hexane (or another hydrophobic solvent such as toluene and chloroform). The product was isolated by centrifugation at 8000 rpm for 5 minutes. Discard the supernatant. Then 10 mL of hexane was added to the precipitate, and the mixture was centrifuged at 8000 rpm for 5 minutes. This washing pr...
Embodiment III
[0061] Example III: Synthesis of Cu nanosheets
[0062] 6.0 mL of OLA (70%) was loaded into a 25 mL three-neck flask, where oxygen was removed by Ar purging for 20 minutes. Then 1.0 mL of TOP (97%) was injected into the flask under Ar flow. After 20 minutes of Ar flow, the flask was rapidly heated to 300°C. Next, 2 mL of Cu-TDA complex solution was quickly injected into the heated flask and the reaction solution turned red. The reaction was held at 300°C for 3 minutes (at least less than 5 minutes). The reaction solution was then cooled to room temperature and injected with 5 mL of hexane (or another hydrophobic solvent such as toluene and chloroform). The product was isolated by centrifugation at 10000 rpm for 5 minutes. Discard the supernatant. Then 5 mL of hexane was added to the precipitate, and the mixture was centrifuged at 10000 rpm for 5 minutes. Repeat the washing procedure twice to remove unreacted precursors and surfactants. Two-dimensional Cu nanosheets wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com