CAR-NK transgenosis carrier based on replication defective recombinant lentivirus, and construction method and application of CAR-NK transgenosis carrier
A technology of recombinant lentivirus and transgenic vector, applied in the field of medical biology, can solve the problems of large lentivirus vector, affecting the infection efficiency of virus immune cells, low recombinant virus titer, etc., achieving good therapeutic effect and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Construction of recombinant lentiviral vectors
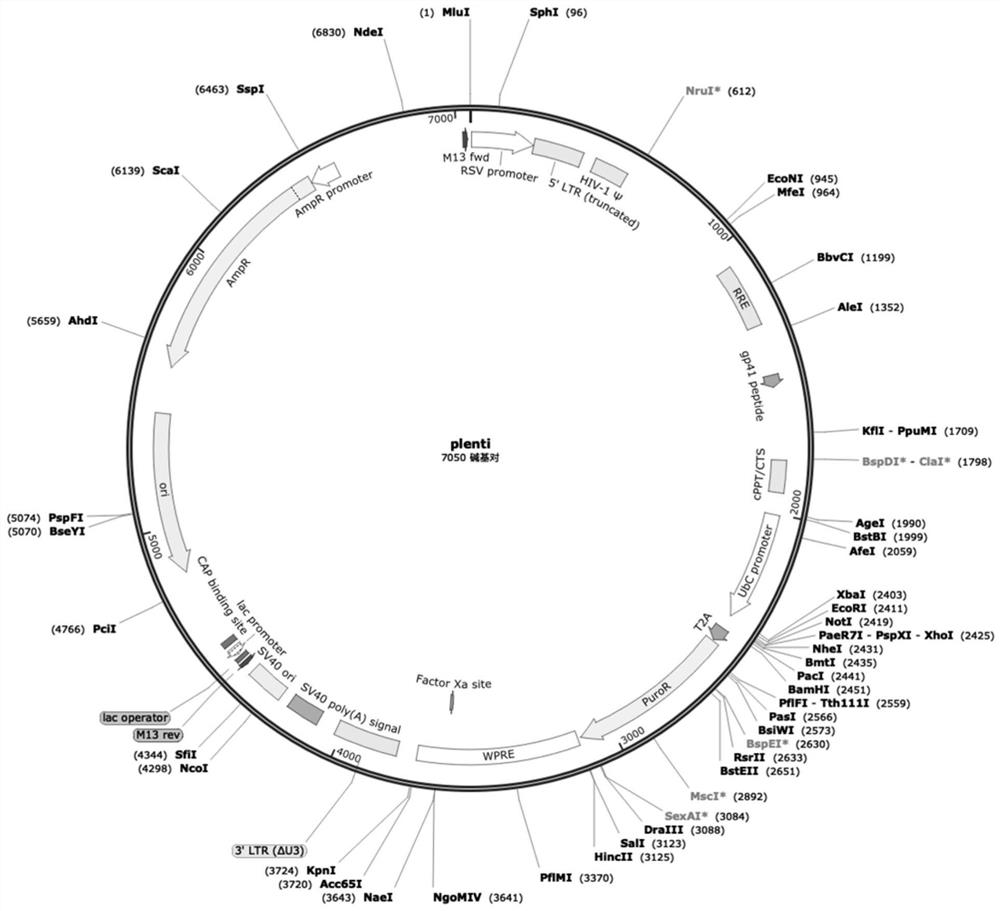
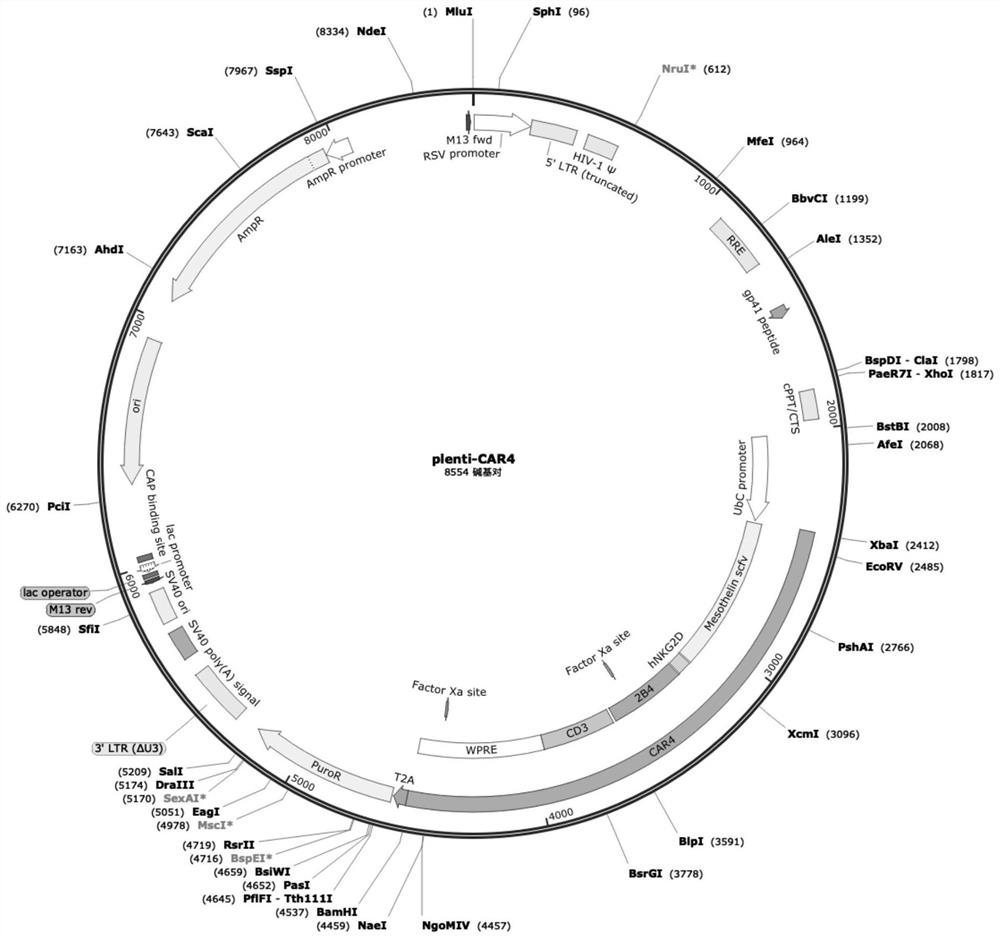
[0035] The Mesothelin single-chain antibody, NKG2D Transmembrane chimeric receptor transmembrane region, 2B4 chimeric receptor co-stimulatory factor, and chimeric receptor activation domain fragments were cloned into the lentiviral backbone plasmid plenti (the size of plenti is 7050bp, and the vector sequence is shown in SEQ ID NO.20, see the attached vector map figure 1 ) to obtain the recombinant lentiviral plasmid plenti-CAR4 (see attached vector map figure 2 ).
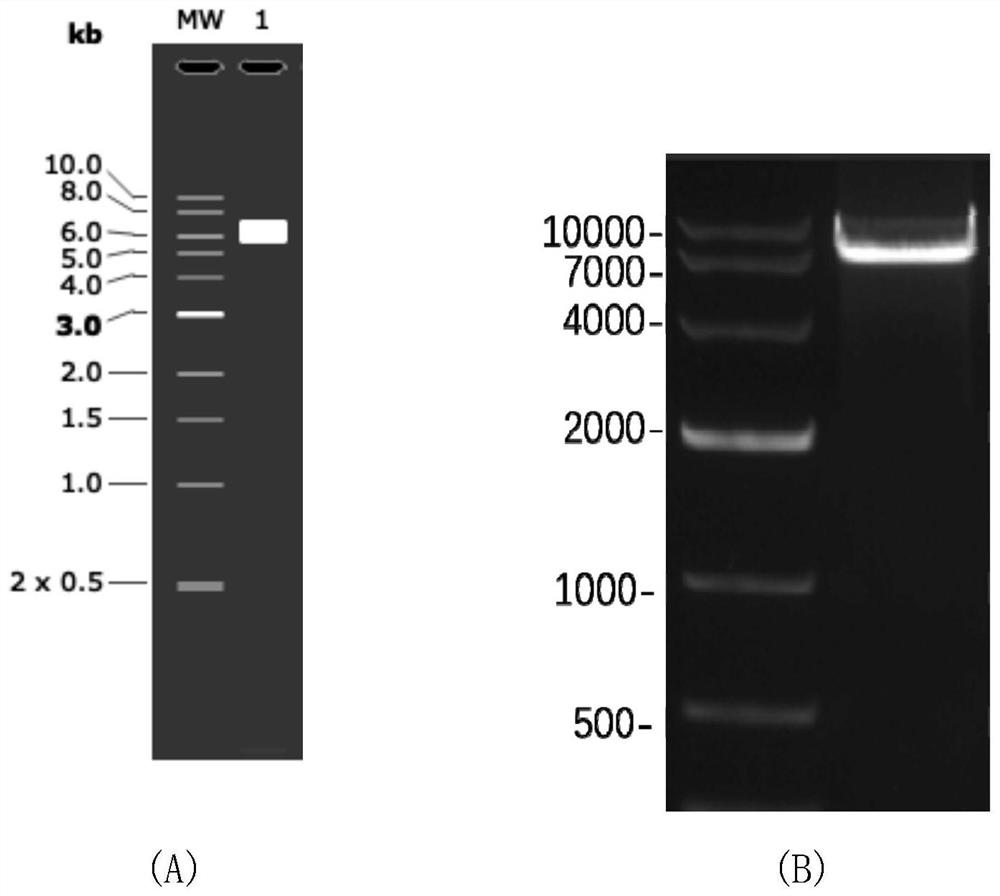
[0036] (1) The lentiviral backbone plasmid plenti was double digested with Xba I and BamH I restriction endonucleases, and the product was subjected to 1.5% agarose gel electrophoresis to confirm the fragment V of 7002bp (such as image 3 shown), and the rubber tapping recovery was placed in an Eppendorf tube, and the corresponding fragment was recovered with an agarose gel recovery kit (steps are shown in Table 1), and the purity and concentration of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com