Cefditoren pivoxil tablet and preparation method thereof
A technology of cefditoren pivoxil and cefditoren, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of sensitivity to light, temperature, humidity, and unfavorable clinical absorption , poor stability and other issues, to achieve the effect of high dissolution rate, simple and flexible operation, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
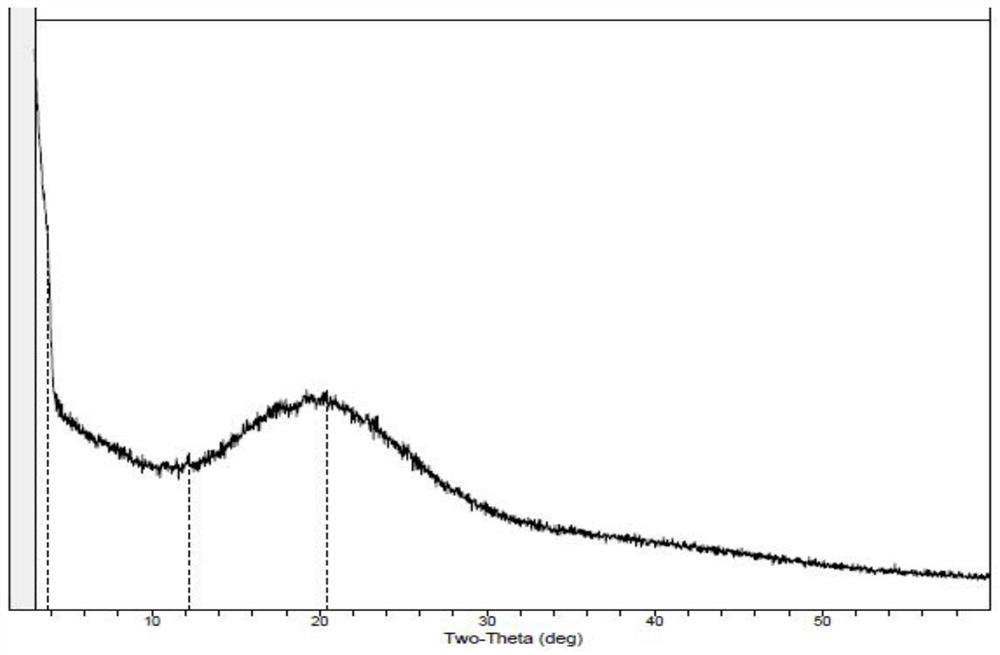
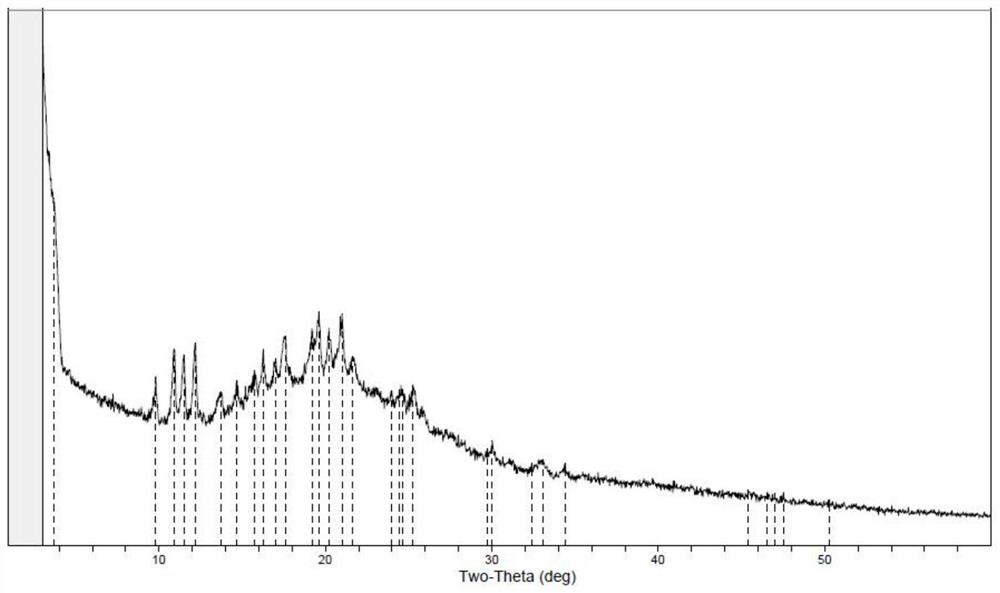
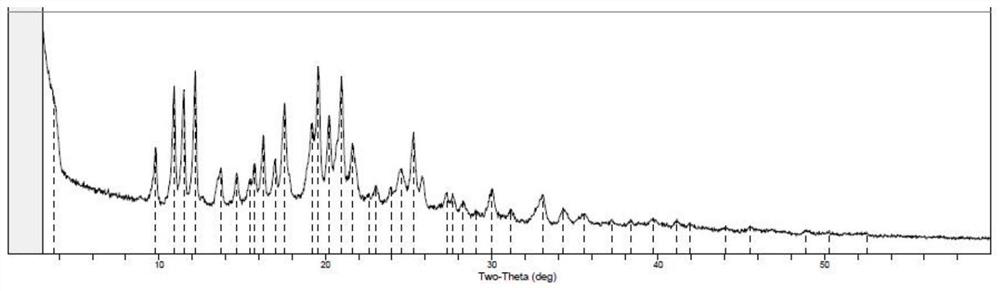
Image
Examples
Embodiment 1
[0061] A cefditoren pivoxil tablet, in parts by weight, comprises the following raw materials:
[0062] Cefditoren pivoxil 100 parts,
[0063] 30 parts of premix,
[0064] Mannitol 60 parts,
[0065] Chitosan 35 parts,
[0066] 10 parts of hydroxypropyl cellulose,
[0067] Glycine 2.5 parts,
[0068] 2.5 parts of talcum powder,
[0069]The premix comprises 66% of low-viscosity polyvinyl alcohol 1788, 10% of trehalose, 10% of lecithin, 8% of mannitol and 6% of titanium dioxide in terms of mass percentage.
[0070] The preparation method of above-mentioned cefditoren pivoxil tablet:
[0071] A. Preparation of acidic hypromellose solution:
[0072] Take about 7L of 1mol / L hydrochloric acid solution, add hypromellose E610g, stir to dissolve completely,
[0073] Cool to 0-5°C and set aside;
[0074] B. At 0-5°C, stir and disperse 1 kg of crystalline cefditoren pivoxil in the acidic hypromellose solution prepared in A until completely dissolved;
[0075] C, the solution th...
Embodiment 2
[0082] A cefditoren pivoxil tablet, in parts by weight, comprises the following raw materials:
[0083] Cefditoren pivoxil 100 parts,
[0084] 30 parts of premix,
[0085] Mannitol 50 parts,
[0086] Chitosan 40 parts,
[0087] 15 parts of hydroxypropyl cellulose,
[0088] Glycine 2 parts,
[0089] 2 parts talcum powder;
[0090] The premix comprises 66% of low-viscosity polyvinyl alcohol 1788, 10% of trehalose, 10% of lecithin, 8% of mannitol and 6% of titanium dioxide in terms of mass percentage.
[0091] The preparation method is the same as in Example 1.
Embodiment 3
[0093] A cefditoren pivoxil tablet, in parts by weight, comprises the following raw materials:
[0094] Cefditoren pivoxil 100 parts,
[0095] 30 parts of premix,
[0096] Mannitol 80 parts,
[0097] Chitosan 20 parts,
[0098] 8 parts of hydroxypropyl cellulose,
[0099] Glycine 5 parts,
[0100] 3 parts talcum powder;
[0101] The premix comprises 66% of low-viscosity polyvinyl alcohol 1788, 10% of trehalose, 10% of lecithin, 8% of mannitol and 6% of titanium dioxide in terms of mass percentage.
[0102] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com