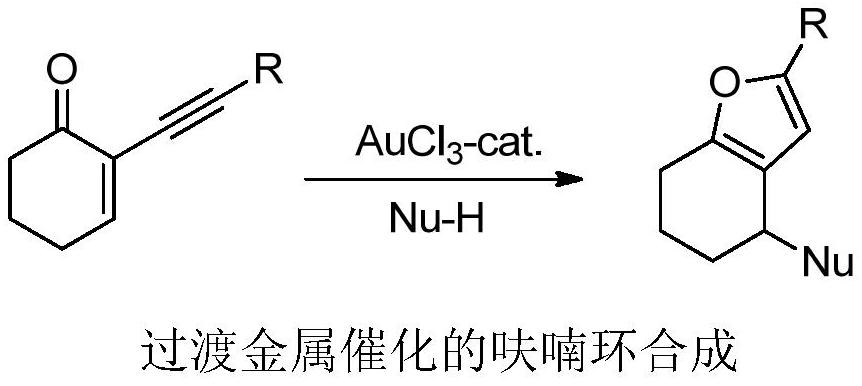
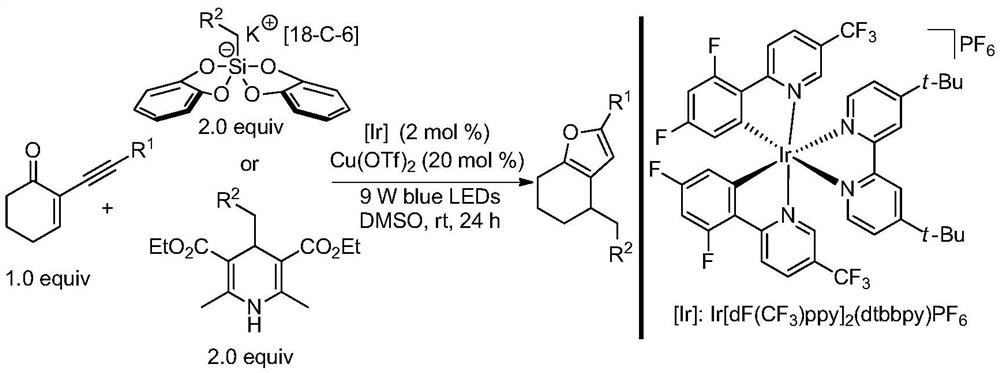
Method for synthesizing polysubstituted furan through photo/copper co-catalysis
A multi-substituted furan and co-catalysis technology is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., to achieve strong functional group compatibility, mild reaction conditions, and substrate Good universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5mg, 0.004mmol, 0.02equiv), 2-(3,3-dimethylbutyne)-2-cyclohexenone (35.2mg, 0.2mmol, 1.0equiv); put the reaction tube into the glove box , Weighed methoxymethyl bis(catechol)silicate-18-crown ether-6-potassium (237.1mg, 0.4mmol, 2.0equiv), copper trifluoromethanesulfonate Cu in the glove box (OTf) 2 (14.4mg, 0.04mmol, 0.2equiv); After weighing, stop the reaction tube with a rubber stopper and take it out, add dry dimethyl sulfoxide DMSO (6mL, 0.033M) to the reaction tube under nitrogen, seal the reaction tube with a parafilm The rubber stopper seals tightly. The reaction tube was irradiated under a 9W blue LED lamp, and after stirring and reacting at room temperature for 24 hours, the light reaction was stopped, and 6 mL of saturated Na 2 CO 3 The solution was stirred for 30min, the aqueous phase after the layers was extracted with ethyl acetate (4×10mL), the organic phases...
Embodiment 2
[0028]
[0029] Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5mg, 0.004mmol, 0.02equiv), 2-phenylacetylene-2-cyclohexenone (39.2mg, 0.2mmol, 1.0equiv); put the reaction tube into the glove box, weigh the ethyl Bis(catechol)silicate-18-crown-6-potassium (230.7mg, 0.4mmol, 2.0equiv), copper trifluoromethanesulfonate Cu(OTf) 2 (14.4mg, 0.04mmol, 0.2equiv); After weighing, stop the reaction tube with a rubber stopper and take it out, add dry dimethyl sulfoxide DMSO (6mL, 0.033M) to the reaction tube under nitrogen, seal the reaction tube with a parafilm The rubber stopper seals tightly. The reaction tube was irradiated under a 9W blue LED lamp, and after stirring and reacting at room temperature for 24 hours, the light reaction was stopped, and 6 mL of saturated Na 2 CO 3 The solution was stirred for 30 min, the aqueous phase after the layers was extracted with ethyl acetate (4×10 mL), the organic phase was washed once with 5 mL of satura...
Embodiment 3
[0031]
[0032] Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5mg, 0.004mmol, 0.02equiv), 2-phenylacetylene-2-cyclohexenone (39.2mg, 0.2mmol, 1.0equiv), 4-isopropylhanstedil (118.1mg, 0.4mmol, 2.0equiv ); Put the reaction tube into the glove box, take trifluoromethanesulfonate copper Cu(OTf) in the glove box 2(14.4mg, 0.04mmol, 0.2equiv); After weighing, stop the reaction tube with a rubber stopper and take it out, add dry dimethyl sulfoxide DMSO (6mL, 0.033M) to the reaction tube under nitrogen, seal the reaction tube with a parafilm The rubber stopper seals tightly. The reaction tube was irradiated under a 9W blue LED light. After stirring and reacting at room temperature for 24 hours, the light reaction was stopped. The reaction solution was added with 6 mL of saturated NaCl solution and stirred for 5 minutes. The separated aqueous phase was extracted with ethyl acetate (4×10 mL) , combined the organic phases with MgSO 4 Dry for 15m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com