A method for electrochemically synthesizing 3-alkylselenyl-4-aminocoumarin compounds
A technology of aminocoumarin and synthesis method, which is applied in the field of electrochemically synthesizing 3-alkylselenyl-4-aminocoumarin compounds, can solve the problem of high cost, serious environmental pollution, and the inability to use 3-alkylselenyl-4 - Issues such as the synthesis of anilinocoumarin compounds, achieving the effects of reducing recovery steps, high reaction selectivity, and facilitating large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
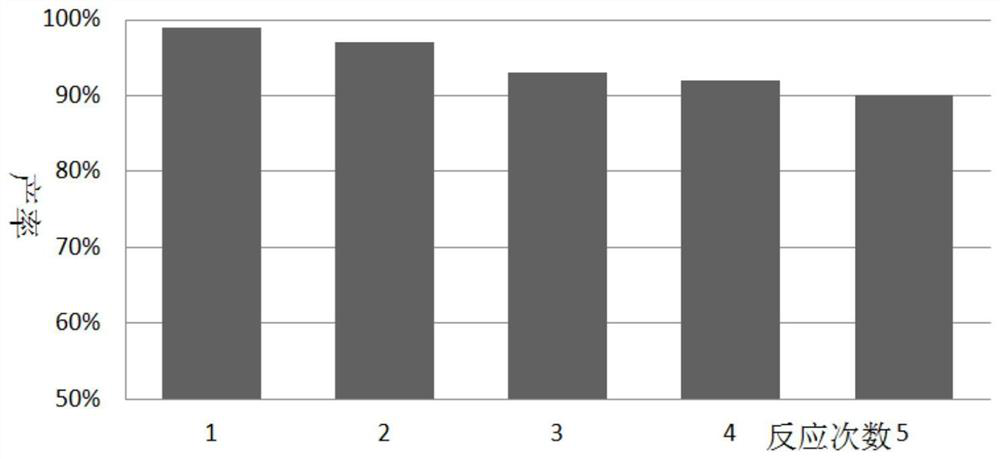
Examples
Embodiment 1~3
[0053] The following examples 1 to 3 are all reacted according to the following reaction equation, mainly to investigate the yield situation of different substrates reacting under optimal conditions:
[0054]
[0055] The specific operation steps are: in a 25mL three-neck round bottom flask, add 4-anilinocoumarin (0.6mmol), dialkyl diselenide (0.3mmol), sodium bromide (0.06mmol), DMF (6mL) successively , 10mm × 10mm × 3mm foam electrode as the anode, 10mm × 10mm × 3mm graphite sheet electrode as the cathode. The resulting mixture was stirred and reacted under a 14 mA direct current at room temperature. The progress of the reaction was followed by thin-layer chromatography, and the reaction time was 4 hours. After the reaction was completed, 6ml of water was added and the product was precipitated, filtered and dried to obtain the pure product.
Embodiment 1
[0057] Compound 1, yield 96%, 3-(methylselanyl)-4-(phenylamino)-2H-chromen-2-one
[0058]
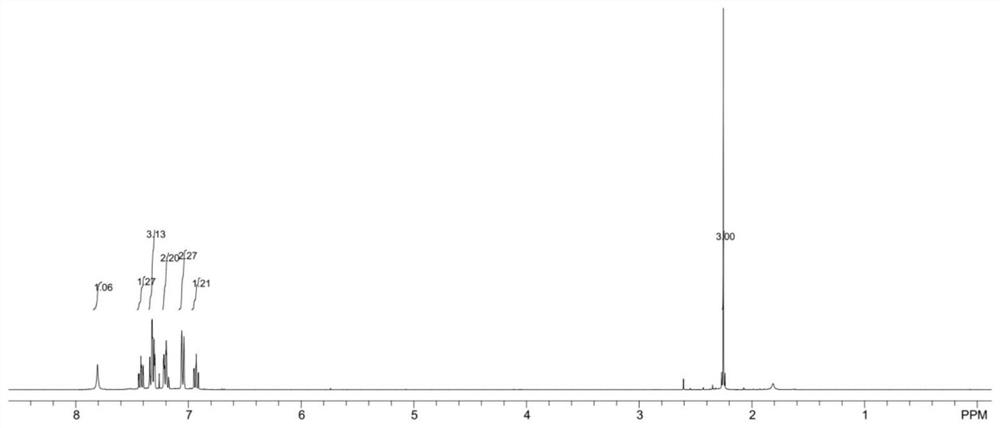
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.81(s,1H),7.44-7.40(m,1H),7.34-7.32(m,3H),7.22-7.17(m,2H),7.05(d,J=8.0Hz,2H),6.90 (t,J=8.4Hz,1H),2.25(s,3H);
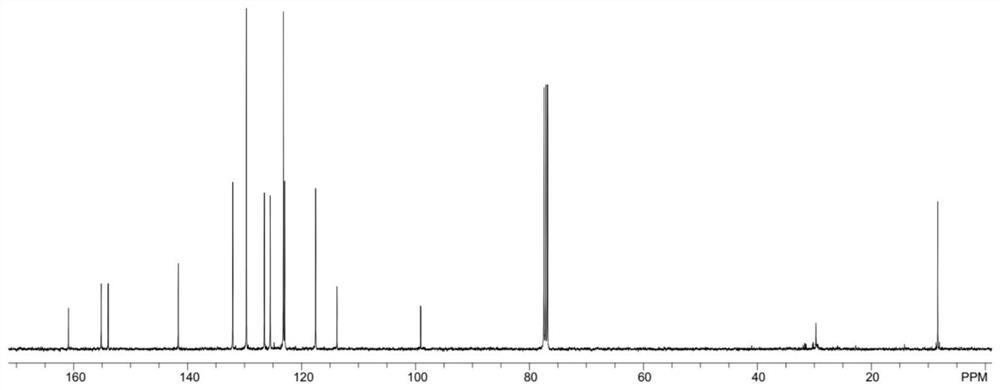
[0060] 13 C NMR (100MHz, CDCl 3 )δ160.9, 155.2, 153.9, 141.6, 132.1, 129.7, 126.5, 125.5, 123.2, 123.0, 117.5, 113.8, 99.1, 8.4;
Embodiment 2
[0062] Compound 2, yield 94%, 3-(benzylselanyl)-4-(phenylamino)-2H-chromen-2-one
[0063]
[0064] 1 H NMR (400MHz, CDCl 3 )δ7.54(s,1H),7.34-7.31(m,1H),7.27-7.23(m,1H),7.16(s,2H),7.12-7.08(m,3H),7.04-7.01(m, 3H),6.97-6.91(m,1H),6.84-6.77(m,1H),6.62(m,2H),4.04(s,2H);
[0065] 13 C NMR (100MHz, CDCl 3 )δ161.4, 156.5, 154.1, 141.1, 139.0, 132.2, 129.4, 128.9, 128.7, 127.1, 126.7, 125.7, 124.0, 123.0, 117.7, 113.6, 96.5, 31.1;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com