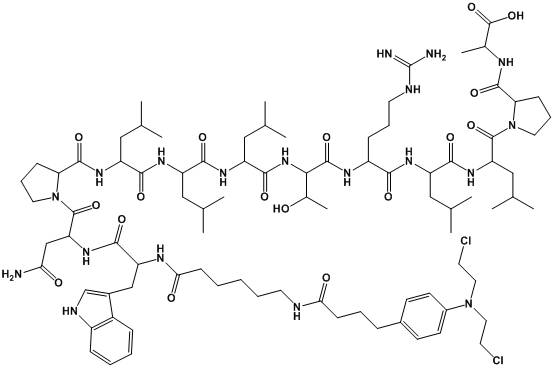
Polypeptide drug conjugate with tumor targeting, and preparation method of polypeptide drug conjugate
A technology of drug conjugates and tumor targeting, which is applied in the field of preparation of polypeptide drug conjugates, can solve the problems of difficulty in the research of anti-tumor polypeptide drug conjugates, and achieve the goal of overcoming toxic side effects and solving core technical problems Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Synthesis of Fmoc-WA1-Wang resin.
[0059] Add Fmoc-Ala-Wang resin (degree of substitution 0.237 mmol / g, 0.711 mmol) to the solid phase reaction tube, then add 25 ml DMF to swell for 20 min. The reagent for removing the Fmoc protecting group was 20% Pip in DMF, and the amino acid condensing agent was HOAt (0.387 g, 2.844 mmol) and DIC (0.440 ml, 2.844 mmol). After adding Fmoc-Pro-OH (0.959 g, 2.844 mmol) to react for 2 h, DMF was washed 3 times to complete the extension of one amino acid. According to the above reaction cycle of amino acid condensation, Fmoc-Leu-OH (1.005 g, 2.844 mmol), Fmoc-Arg(pbf)-OH (1.845 g, 2.844 mmol), Fmoc-Thr(tBu )-OH (1.130 g, 2.844 mmol), Fmoc-Asn(Trt)-OH (1.697 g, 2.844 mmol), Fmoc-Trp(Boc)-OH (1.498 g, 2.844 mmol) were reacted and washed twice with DMF , and washed twice with DCM, and dried to obtain Fmoc-Trp(Boc)-Asn(Trt)-Pro-Leu-Leu-Leu-Thr(tBu)-Arg(pbf)-Leu-Leu-Pro-Ala-Wang , namely Fmoc-WA1-Wang resin.
Embodiment 2
[0060] Example 2 Synthesis of Fmoc-linker-WA1-Wang resin.
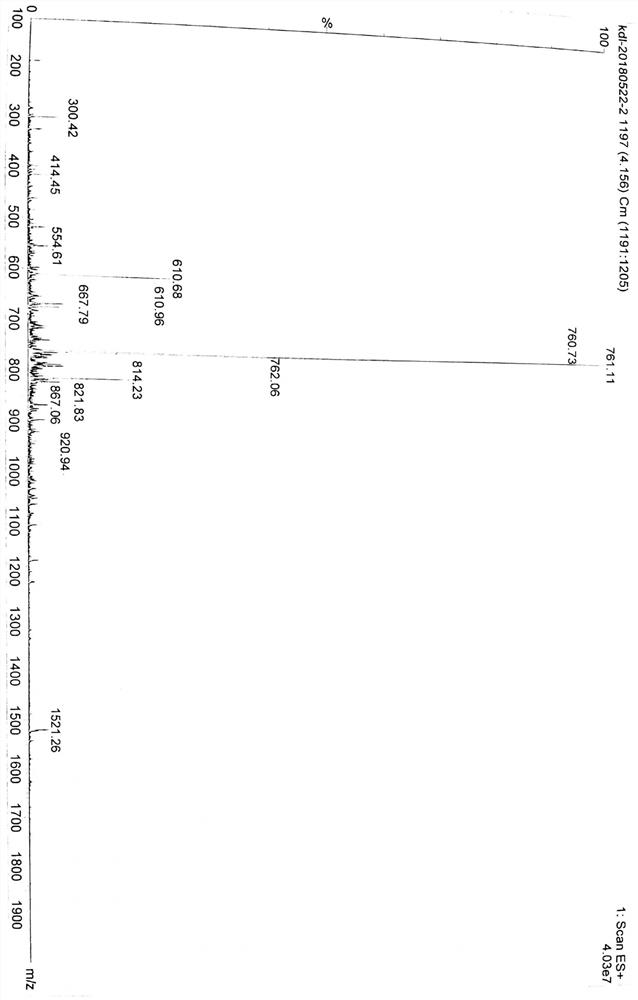
[0061] The obtained Fmoc-WA1-Wang resin was deprotected by 20% Pip in DMF, and then HOAt (0.387 g, 2.844 mmol), DIC (0.440 ml, 2.844 mmol) and Fmoc-Acp-OH (1.005 g, 2.844 mmol) was reacted in an ultrasonic reactor for 45 min, and the reaction end point was judged by Kaiser detection, the reaction solution was drained, and washed twice with DMF to obtain Fmoc-linker-WA1-Wang resin. (A small amount of product was treated with a peptide cleavage reagent to obtain WA1-lineker, which was detected by mass spectrometry, and the results are shown in figure 1 .
Embodiment 3
[0062] Example 3 Synthesis of PDC-WA1.
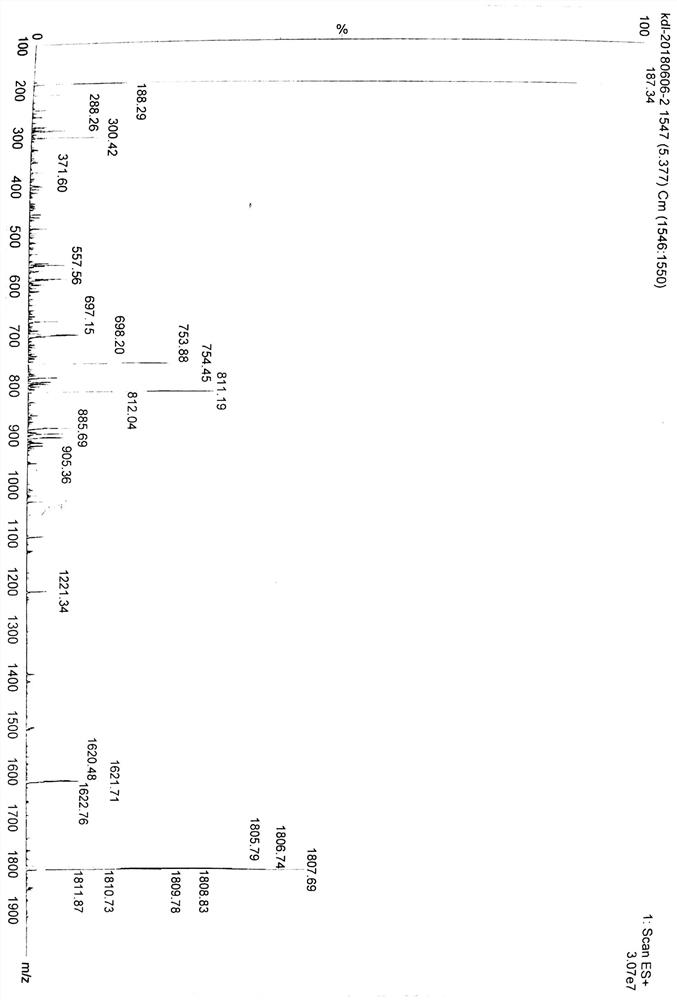
[0063] Also use 20% Pip in DMF solution to remove the Fmoc protecting group of Fmoc-WA1-Wang resin, add HOAt (0.387 g, 2.844 mmol), DIC (0.440 ml, 2.844 mmol) and chlorambucil (0.865 g, 2.844 mmol) mmol) were reacted in an ultrasonic reactor for 1 h, washed twice with DMF, and the resin was shrunk with methanol. Add peptide cleavage reagent 95% TFA aqueous solution, react under ice bath for 1.5h. Filter, add the filtrate to anhydrous ether, and centrifuge to obtain the crude peptide. The crude peptide was dissolved, purified by HPLC, and then freeze-dried to obtain a product of 0.449 g (yield: 35%), which was detected by mass spectrometry. The results are shown in figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com