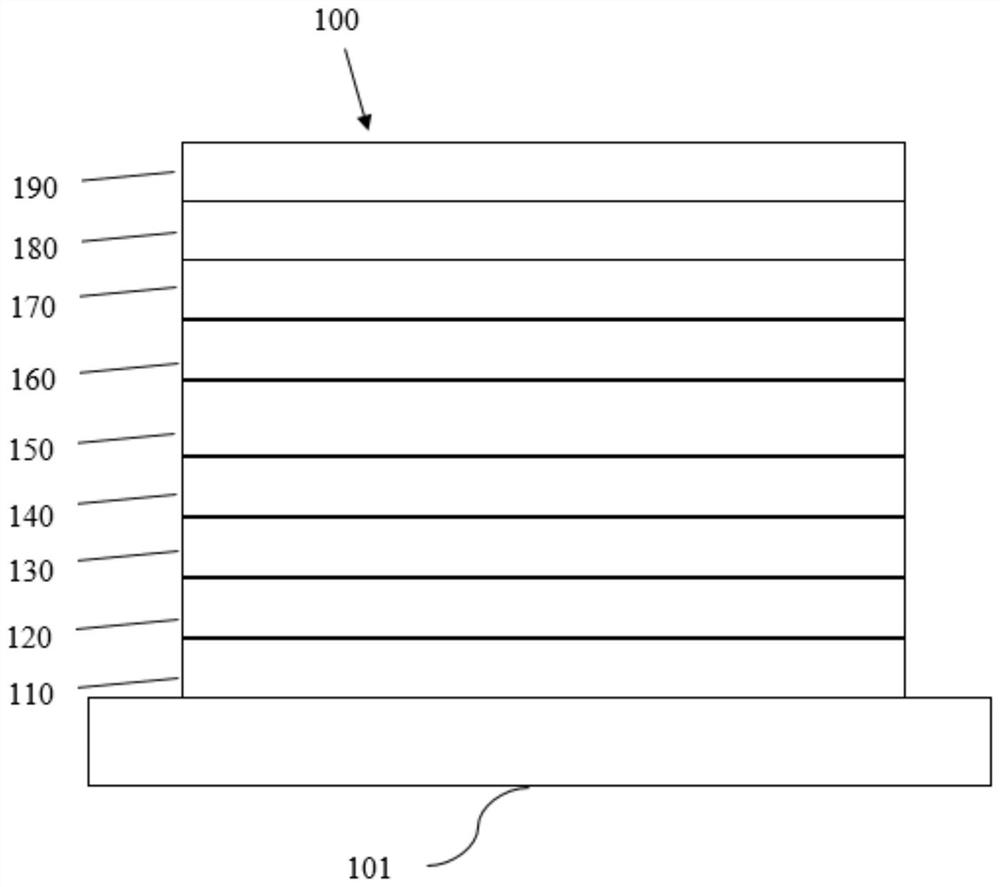
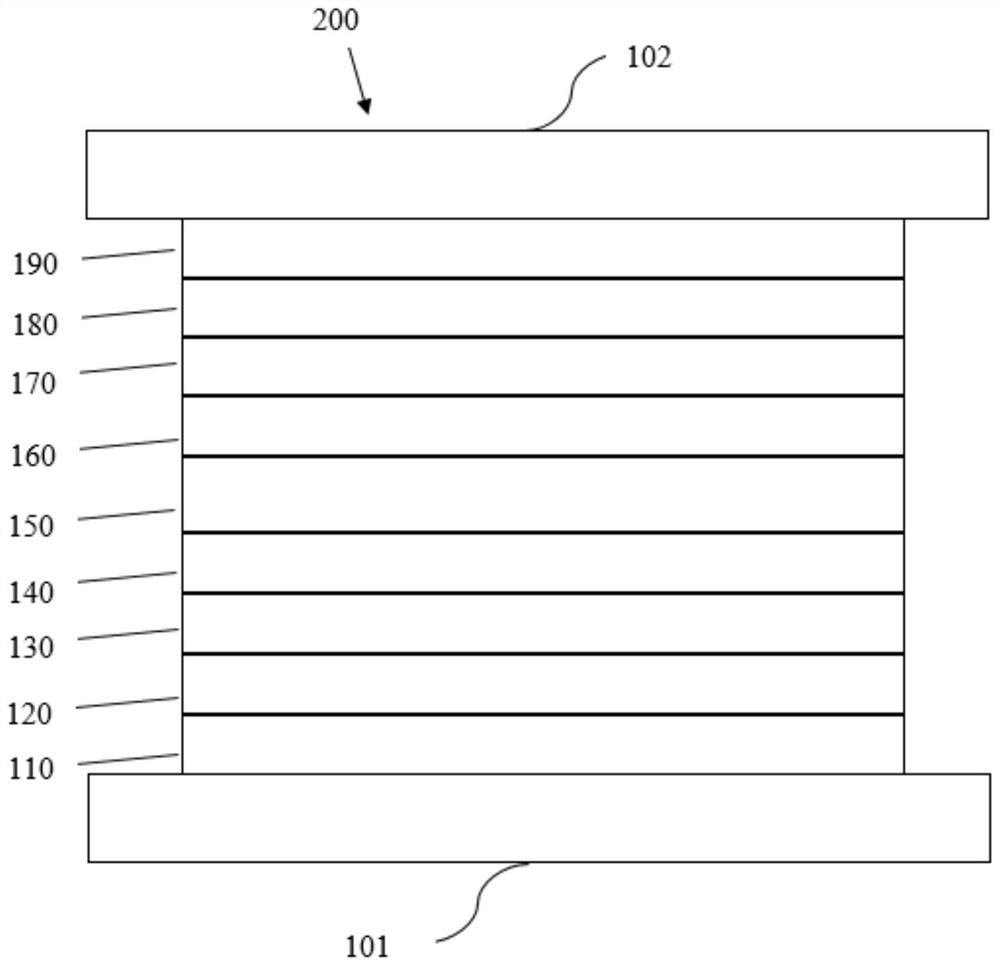
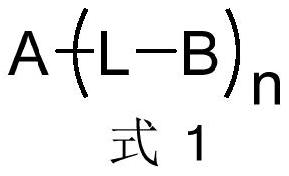Electron transport material containing azaspirobifluorene
A technology of carbon atoms and heteroaryl groups, which is applied in the field of electron transport materials containing nitrogen-containing spirobifluorene, can solve the problems of undisclosed or taught pyrimidine/triazine structural fragments, etc., and achieve the effect of long device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0153] Embodiment: the synthesis of compound 87
[0154] Step 1: Synthesis of [Intermediate a]
[0155]
[0156] Methyl 2,5-dichloronicotinate (11.8g, 57.3mmol), phenylboronic acid (7.08g, 57.9mmol), bistriphenylphosphine palladium dichloride (2.25g, 3.21mmol), potassium carbonate (16.4 g, 118.7mmol) into a 500mL three-necked round-bottomed flask, and then 225mL of 1,4-dioxane and 50mL of water were added. The reaction flask was heated to reflux at 80°C and stirred for 12 hours under nitrogen atmosphere. After the reaction, the reaction system was cooled to room temperature and extracted with ethyl acetate, and the organic phase was dried and concentrated and separated by column chromatography (petroleum ether: ethyl acetate) to obtain a colorless oil [Intermediate a] (12.3g, 49.7mmol , 86.7%).
[0157] Step 2: Synthesis of [Intermediate b]
[0158]
[0159] Add [Intermediate a] (12.3g, 49.7mmol) into a 250mL three-neck round bottom flask, then add methanol 50mL, sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com