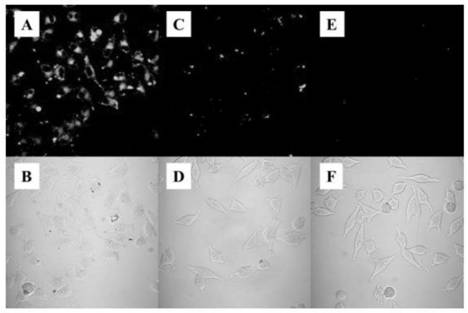
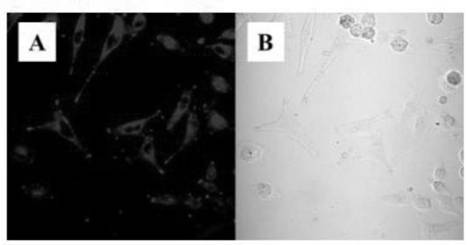
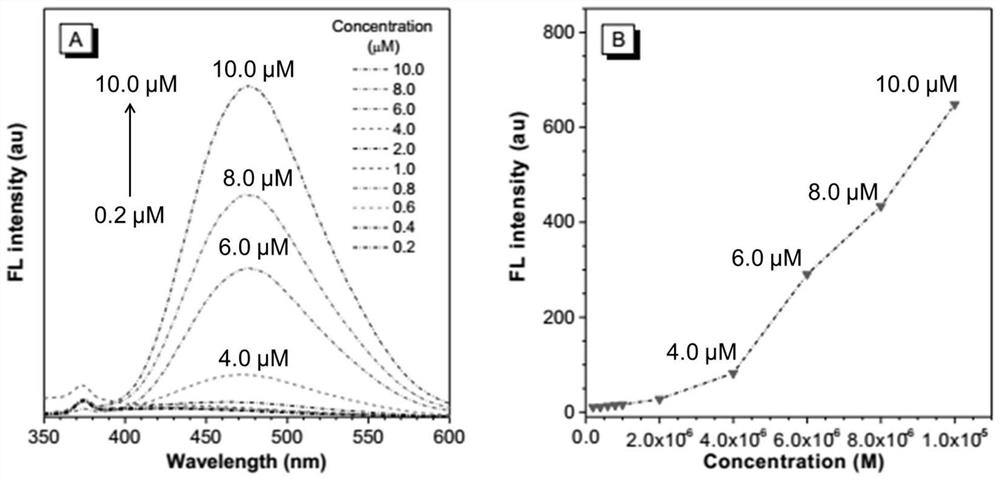
Peptide chain modified tetraphenylethylene derivative and preparation method and application thereof
A technology of tetraphenylethylene and derivatives, applied in the field of fluorescence imaging, can solve the problems of harmful organisms, cumbersome process, difficult modification of nanoparticles, and achieve good biocompatibility, good selective permeability, and increased solubility. and cell permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] In the present invention, when R is -H, the preparation method of the tetraphenylethylene derivatives modified by the peptide chain comprises the following steps:
[0058] Mix 4-(1,2,2-triphenylethenyl)benzaldehyde, a polypeptide containing cysteine residues, and N,N-dimethylformamide for a nucleophilic addition-cyclization reaction, Obtain peptide chain-modified tetraphenylethylene derivatives;
[0059] Wherein, the structural formula of the polypeptide containing cysteine residues is as follows:
[0060]
[0061] The reaction scheme is as follows:
[0062]
[0063] In the present invention, the molar ratio of the 4-(1,2,2-triphenylvinyl)benzaldehyde to the polypeptide containing cysteine residues is preferably 1:(1~1.5), more preferably 1: (1~1.2). In the present invention, the N,N-dimethylformamide (DMF) is used as a solvent, and the dosage ratio of the N,N-dimethylformamide and the polypeptide containing cysteine residues is preferably 2 mL: (0.033...
Embodiment 1
[0097] Preparation of tetraphenylethylene derivatives (referred to as compound I-1-i) modified with a peptide chain having a structure shown in formula I-1-i comprises the following steps:
[0098] Dissolve the polypeptide containing cysteine residues (Peptidyl has the structure shown in formula i, 0.033mmol) and 4-(1,2,2-triphenylethenyl)benzaldehyde (0.04mmol) in N,N- In dimethylformamide (DFM, 2mL), the resulting mixture was stirred and reacted at room temperature (25°C) for 24h; after the reaction was completed, the resulting product system was slowly added to anhydrous ether (200mL) and allowed to stand for 12h A precipitate was precipitated, and then the filter cake was collected by filtration, washed with anhydrous ether, and dried to obtain the target product as a yellow solid.
[0099] Described target product is characterized, and the result is as follows:
[0100] MALDI-TOF:[M+H] + m / z 1356.7291 (found), 1356.7285 (calculated).
[0101] From the above molecular...
Embodiment 2
[0103] Preparation of a peptide chain modified tetraphenylethylene derivative (referred to as compound I-1-ii) having a structure shown in formula I-1-ii comprises the following steps:
[0104] Dissolve the polypeptide containing cysteine residues (Peptidyl has the structure shown in formula ii, 0.036mmol) and 4-(1,2,2-triphenylethenyl)benzaldehyde (0.04mmol) in N,N- In dimethylformamide (2mL), the resulting mixture was stirred and reacted at room temperature for 24h; after the reaction, the resulting product system was slowly added to anhydrous ether (150mL), and left to stand for 12h to precipitate a precipitate, which was then collected by filtration The filter cake was washed with anhydrous ether and dried to obtain the target product as a yellow solid.
[0105] Described target product is characterized, and the result is as follows:
[0106] MALDI-TOF:[M+H] + m / z 1761.6886 (found), 1761.6869 (calculated).
[0107] From the above molecular weight data, it can be seen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com