Trisome syndrome gene nucleic acid detection quality control product based on CRSIPR-Cas9 and organoid culture and preparation method therefor
A technology for trisomy syndrome and organoids, which is applied in the field of trisomy gene nucleic acid detection quality control products and its preparation, can solve the problems of large-scale clinical application and uneven precision, and achieve high genome stability, The effect of long storage time and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention provides a method for preparing a trisomy gene nucleic acid detection quality control product based on CRSIPR-Cas9 and organoid culture, and the preparation steps are as follows:
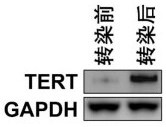
[0038] (1) Using CRISPR-Cas9 technology to knock-in and express telomerase TERT in peripheral blood lymphocytes derived from patients with trisomy 13, 18, and 21, to construct gene-edited lymphocytes that can stably expand and divide; the specific steps are as follows: :
[0039] Design 3 pairs of specific primers based on the full-length domain of the TERT gene, and amplify the relevant fragments to cover the entire CDS region of the TERT gene. The primers are shown in the table below (Table 1). After the amplification was completed, the three parts of the sequence amplified by the primers in Table 1 were integrated into a single Puro expression plasmid, and the expression donor plasmid with 800bp homology arms was inserted into the two segments respectively. Introduce CRISPR-...
Embodiment 1
[0056] Accuracy identification of quality control products for trisomy 13, 18, and 21 by next-generation sequencing
[0057] Use digital PCR technology to test different proportions of quality control products to identify their accuracy. The result is as Figure 5 As shown, in each order of magnitude, the digital PCR test results have a significant correlation with the expected mixing ratio, and the correlation coefficient is >0.95, confirming that the quality control product has a high degree of accuracy.
Embodiment 2
[0059] Reproducibility identification of quality control products for trisomy 13, 18, and 21 by next-generation sequencing
[0060] Full levels (1:10) prepared from three different batches using digital PCR technology 3 , 1:10 4 , 1:10 5 , 1:10 6 , 1:10 7 ) Quality control products are tested to identify the repeatability of different batches of products. The result is as Figure 6 As shown, there is no significant difference in the detection values of the three different batches at all levels, and they are kept in a good SD range, which proves that the preparation process of this product has excellent repeatability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com