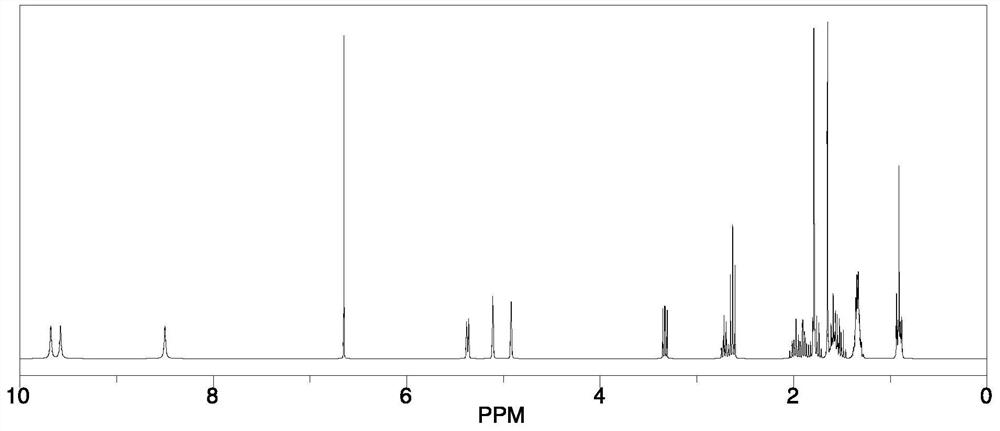
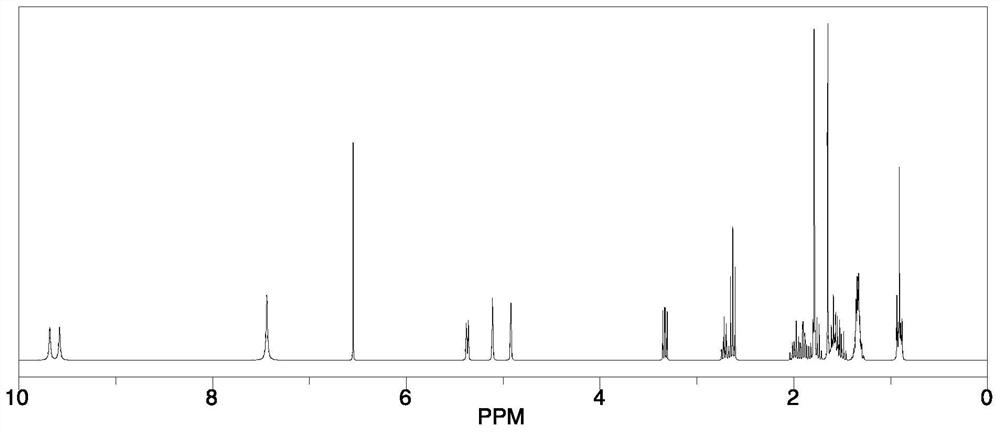
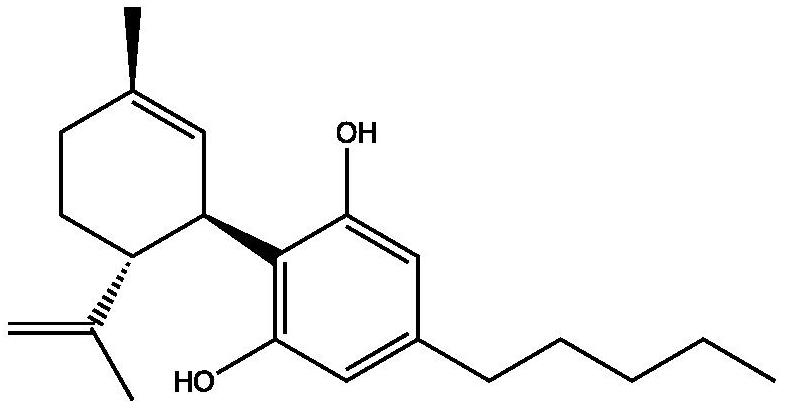
A kind of cannabidiol-3-sulfonic acid and its preparation method and application, cannabidiol derivative
A technology of cannabidiol and derivatives, which is applied in the field of medicine and chemical industry to achieve the effects of broadening the research and application scope, increasing water solubility, and increasing drugability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides the preparation method of cannabidiol-3-sulfonic acid described in the above technical scheme, comprising the following steps:
[0033] Mix cannabidiol with concentrated sulfuric acid for sulfonation reaction to obtain cannabidiol-3-sulfonic acid.
[0034] In the present invention, unless otherwise specified, the required preparation materials are commercially available products well known to those skilled in the art.
[0035] The invention mixes cannabidiol and concentrated sulfuric acid to carry out sulfonation reaction to obtain cannabidiol-3-sulfonic acid. In the present invention, the concentrated sulfuric acid is preferably commercially available concentrated sulfuric acid, that is, concentrated sulfuric acid with a mass fraction of 98%.
[0036] In the present invention, the molar ratio of cannabidiol to concentrated sulfuric acid is preferably 1:15-30, more preferably 1:20-25. In the present invention, the mixing process is prefe...
Embodiment 1
[0055] The ratio of cannabidiol to concentrated sulfuric acid molar ratio is 1:15 to react.
[0056] Weigh 50g of concentrated sulfuric acid and add it to a three-necked flask, cool it down to 0°C naturally in an ice-water bath, add 10.68g of cannabidiol, control the temperature below 20°C, after the addition is complete, then control the temperature at 20°C, carry out the sulfonation reaction for 4 hours, and stop the reaction. Pour the resulting reaction liquid into an ice-water bath, and carry out ethyl acetate extraction. The extracted organic phase was concentrated under reduced pressure at 50°C and then placed on a 200-300 mesh silica gel column. =8:1:1) to elute 2 column volumes, then change to eluent 2 (petroleum ether:ethyl acetate:methanol=4:1:2) to elute 3 column volumes, collect eluent 2 to wash Partially removed, after drying under reduced pressure, add an appropriate amount of methanol at room temperature until the solids are completely dissolved, let stand at ro...
Embodiment 2
[0060] The reaction is carried out in a ratio of 1:30 by the molar ratio of cannabidiol and concentrated sulfuric acid.
[0061] Weigh 50g of concentrated sulfuric acid and add it to a three-necked flask, cool it down to 0°C naturally in an ice-water bath, add 5.34g of cannabidiol, control the temperature below 15°C, after the addition is complete, then control the temperature at 25°C, carry out the sulfonation reaction for 2 hours, and stop the reaction. Pour the resulting reaction solution into an ice-water bath, extract with ethyl acetate, dry the extracted organic phase under reduced pressure at 50°C, dissolve it in 10% ethanol solution, and load it on an AB-8 macroporous adsorption resin column , first wash 5 column volumes with 10% ethanol, then wash 3 column volumes with 50% ethanol, and finally wash the column with 3 column volumes of 95% ethanol; collect 50% ethanol eluted parts, dry under reduced pressure Add an appropriate amount of absolute ethanol until the solid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com