Crystal form and preparation method of clomipamine hydrochloride
A technology of clomipramine hydrochloride and crystal form, which is applied in the field of drug crystal form, can solve the problems of no clomipramine hydrochloride, etc., and achieve the effect of stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
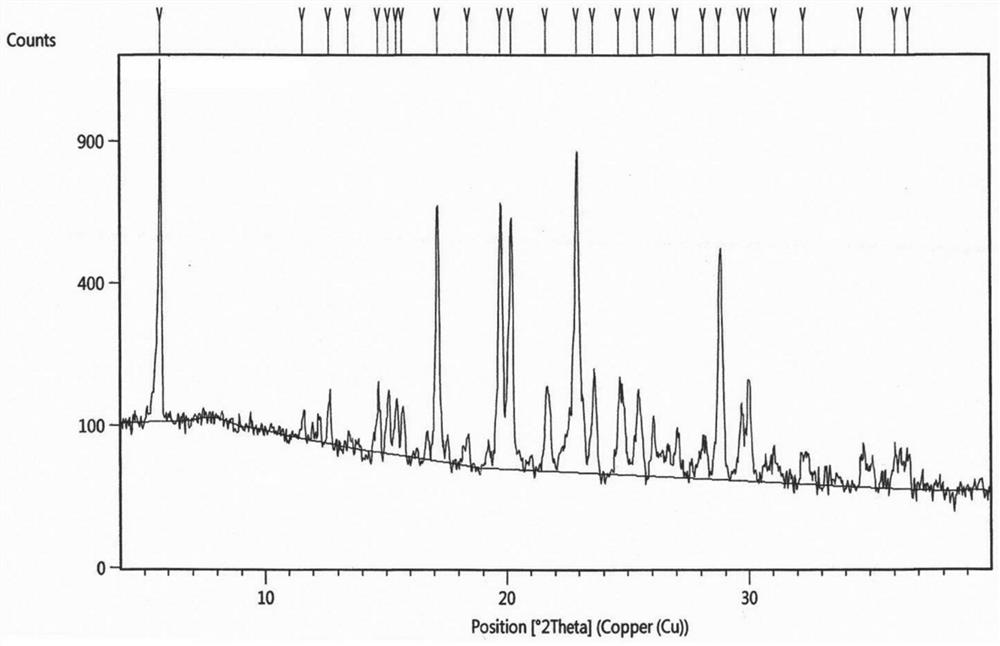
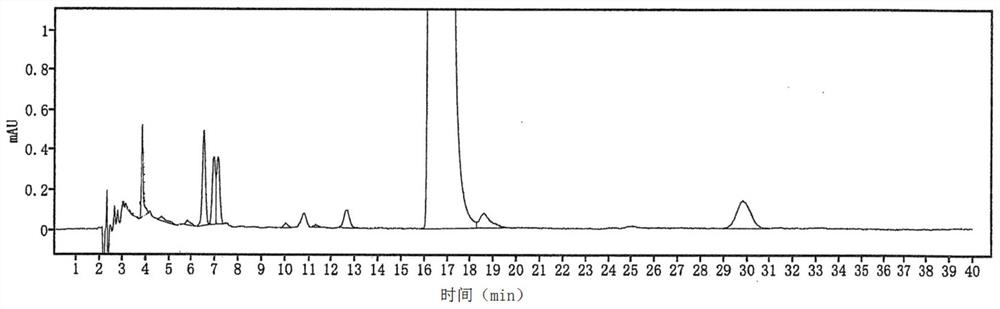
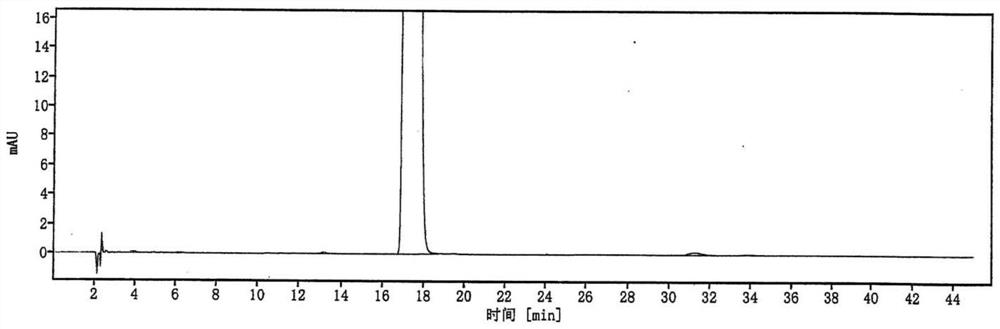
Image
Examples
Embodiment 1
[0028] Preparation route of the present invention:
[0029] Using the commercially available sangite of formula I as the starting material, the hydrolyzate of formula II is obtained by hydrolysis under alkaline conditions (refer to existing patents). The difference in this process is that the commercially available side chain (N, N-dimethyl chloropropane hydrochloride) to prepare the formula III condensate, the formula III condensate is formed into a salt by feeding HCl gas into the toluene solution to prepare clomipramine hydrochloride.
[0030] (1) Preparation of hydrolyzate
[0031]
[0032] (2) Preparation of condensate
[0033]
[0034] (3) Preparation of crude product
[0035]
[0036] (4) Preparation of high-quality goods
[0037]
[0038] Step (1) and step (2) of the present invention are the same as the prior art (for example, the patent application number is CN202010632533.7), step (3) is, 50g clomipramine is added in 250g toluene, then pass through I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com