Method for synthesizing cytidine
A technology of cytidine nucleoside and tetraacetyl ribose, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of unsuitable industrial production, cytosine is easy to deliquescence, difficult to obtain, etc., and achieves the design route Novelty, simple process operation and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
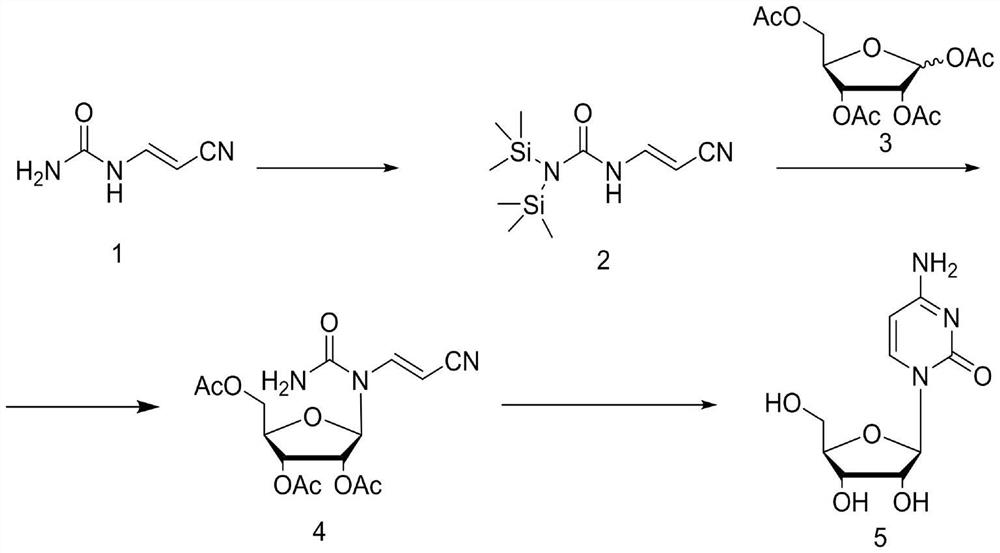
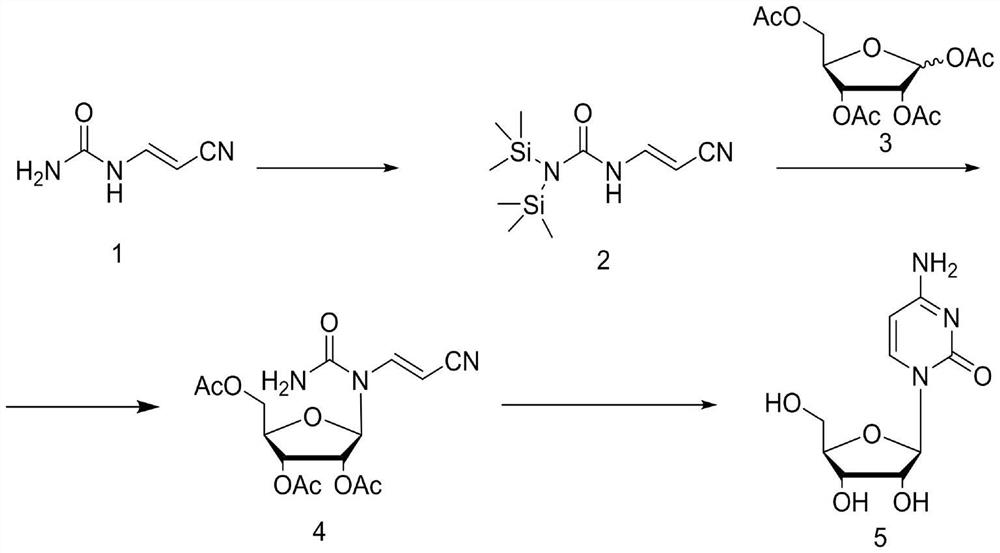
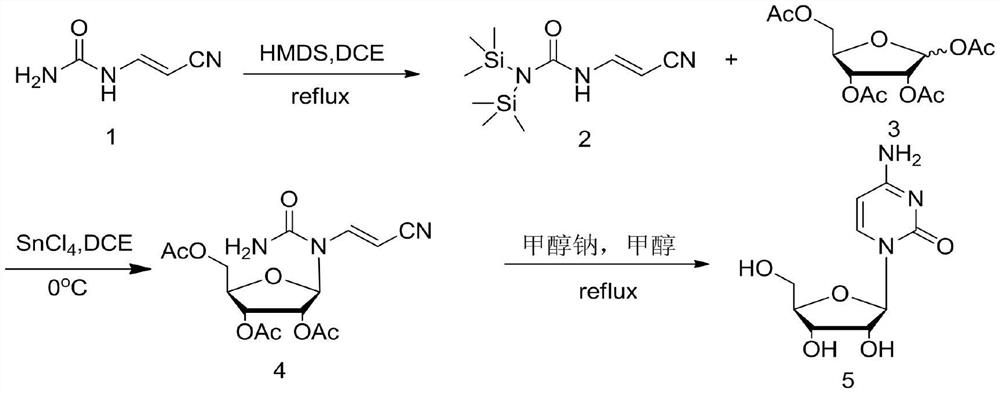
[0028] The first step: add cyanoacetaldehyde urea 1 (22g, 0.2mol) and dichloroethane 300mL into the three-necked flask and mix well, then add hexamethyldisilazane (19.4g, 0.12mol), 5 % Catalyst ammonium sulfate (1.32g, 0.01mol), heat up to reflux for 5h, the reaction liquid dissolves, and the raw materials can be directly put into the next reaction after the reaction is complete.
[0029] The second step: add tetraacetyl ribose 3 (66.84g, 0.21mol) to the reaction solution of the previous step, after stirring and dissolving, the reaction solution is cooled to -5°C, and tin tetrachloride (26g, 0.1mol) is slowly added dropwise to keep The temperature of the reaction solution was below 5°C. After the dropwise addition, the temperature was raised to 35°C and reacted for 3 hours. TLC tracked until the raw materials disappeared, and then the reaction solution was cooled to -5°C, and saturated sodium bicarbonate was slowly added dropwise to quench the reaction. Then extrac...
Embodiment 2
[0032]
[0033] The first step: add cyanoacetaldehyde urea 1 (22g, 0.2mol) and hexamethyldisilazane (84g, 0.52mol) in the there-necked flask and mix well, then add 5% catalyst ammonium sulfate (1.32g , 0.01mol), the temperature was raised to reflux for 5 hours, the reaction solution was dissolved and the raw materials were completely reacted. The reaction solution was concentrated under reduced pressure to obtain hexamethyldisilazane, and 300 mL of methylene chloride was added.
[0034] The second step: add tetraacetylribose 3 (67.4g, 0.21mol) to the solution of the previous step, after stirring and dissolving, the reaction solution is cooled to -5°C, and tin tetrachloride (28.6g, 0.11mol) is slowly added dropwise to keep The temperature of the reaction solution was below 5°C. After the dropwise addition, the temperature was raised to 35°C and reacted for 3 hours. TLC tracked until the raw materials disappeared, and then the reaction solution was cooled to -5°C, and saturate...
Embodiment 3
[0037]
[0038] The first step: add cyanoacetaldehyde urea 1 (22g, 0.2mol) and dichloroethane 300mL in the there-necked flask and stir and mix evenly, trimethylchlorosilane (33.2g, 0.22mol), triethylamine ( 22.3g, 0.22mol), heated to reflux for 5h. The temperature of the reaction solution was lowered to 0°C, and triethylamine hydrochloride was removed by filtration, and the filtrate could be directly put into the next reaction.
[0039] The second step: add tetraacetylribose 3 (76.39g, 0.24mol) to the filtrate of the previous step, stir and dissolve, then cool the reaction solution to -5°C, and slowly drop tin tetrachloride (26g, 0.1mol) to maintain the reaction The temperature of the liquid is below 5°C. After the dropwise addition, the temperature is raised to 35°C and reacted for 3 hours. TLC traces until the raw materials disappear, and then the reaction liquid is cooled to -5°C, and saturated sodium bicarbonate is slowly added dropwise to quench the reaction. Extract ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com