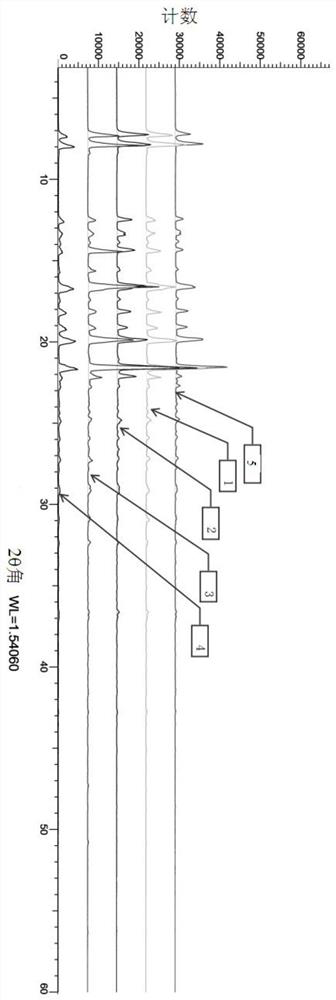
Crystallization process of dexibuprofen
A technology of dexbuprofen and crystallization, which is applied in the field of crystallization technology of dexbuprofen, can solve the problems of inability to accurately control quality, crystal form and particle size, and is not suitable for large-scale production, and achieves that key parameters can be accurately controlled , stable and reliable quality, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The crystallization process of the Dexibuprofen of the present embodiment comprises the following steps:
[0048] Step 1: Add 25Kg of crude dextrobuprofen and 75Kg of acetone to the reaction kettle, stir and heat up to 35°C, after the materials are completely dissolved, filter, wash the filter cake with a little acetone, and concentrate the acetone from the filtrate to dryness under reduced pressure to obtain a white solid .
[0049] Step 2, add 2.5Kg of acetone to the white solid of the concentrate, stir and raise the temperature to 50°C, after the material is completely dissolved, control the stirring speed at 60 rpm, and start to cool down slowly, when the internal temperature is 30°C, the material begins to precipitate;
[0050] Step 3, after observing the precipitation of crystalline materials, keep the temperature and stirring speed unchanged for 0.5h, control the vacuum degree to 0.95atm, and slowly distill out acetone;
[0051] Step 4, until the material is all...
Embodiment 2
[0053] The crystallization process of the Dexibuprofen of the present embodiment comprises the following steps:
[0054] Step 1: Add 25Kg of crude product of Dexibuprofen and 75Kg of acetone into the reaction kettle, stir and heat up to 35°C, after the materials are completely dissolved, filter, wash the filter cake with a little acetone, and concentrate the filtrate to dryness under reduced pressure to obtain a white solid.
[0055] Step 2: Add 2.0Kg of acetone to the white solid, stir and raise the temperature to 50°C. After the material is completely dissolved, control the stirring speed to 80 rpm, and start to cool down slowly. When the internal temperature is 36°C, crystals of the material begin to precipitate;
[0056] Step 3: After observing crystal precipitation, keep the temperature and stirring speed unchanged for 0.5h, control the vacuum degree to 0.90 atm, and slowly distill out acetone;
[0057] Step 4, until the material is all converted into white crystals, turn...
Embodiment 3
[0059] The crystallization process of the Dexibuprofen of the present embodiment comprises the following steps:
[0060] Step 1: Add 25Kg of crude product of Dexibuprofen and 75Kg of acetone into the reaction kettle, stir and heat up to 35°C, after the materials are completely dissolved, filter, wash the filter cake with a little acetone, and concentrate the filtrate to dryness under reduced pressure to obtain a white solid.
[0061] Step 2: Add 1.5Kg of acetone to the white solid, stir and heat up to 50°C, after the material is completely dissolved, control the stirring speed to 40 rpm, and start to slowly cool down, when the internal temperature is 43°C, the material begins to precipitate;
[0062] Step 3: After observing crystal precipitation, keep the temperature and stirring speed unchanged for 0.5h, control the vacuum degree to 0.90 atm, and slowly distill out acetone;
[0063] Step 4, until the material is all converted into white crystals, turn on the vacuum, continue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com