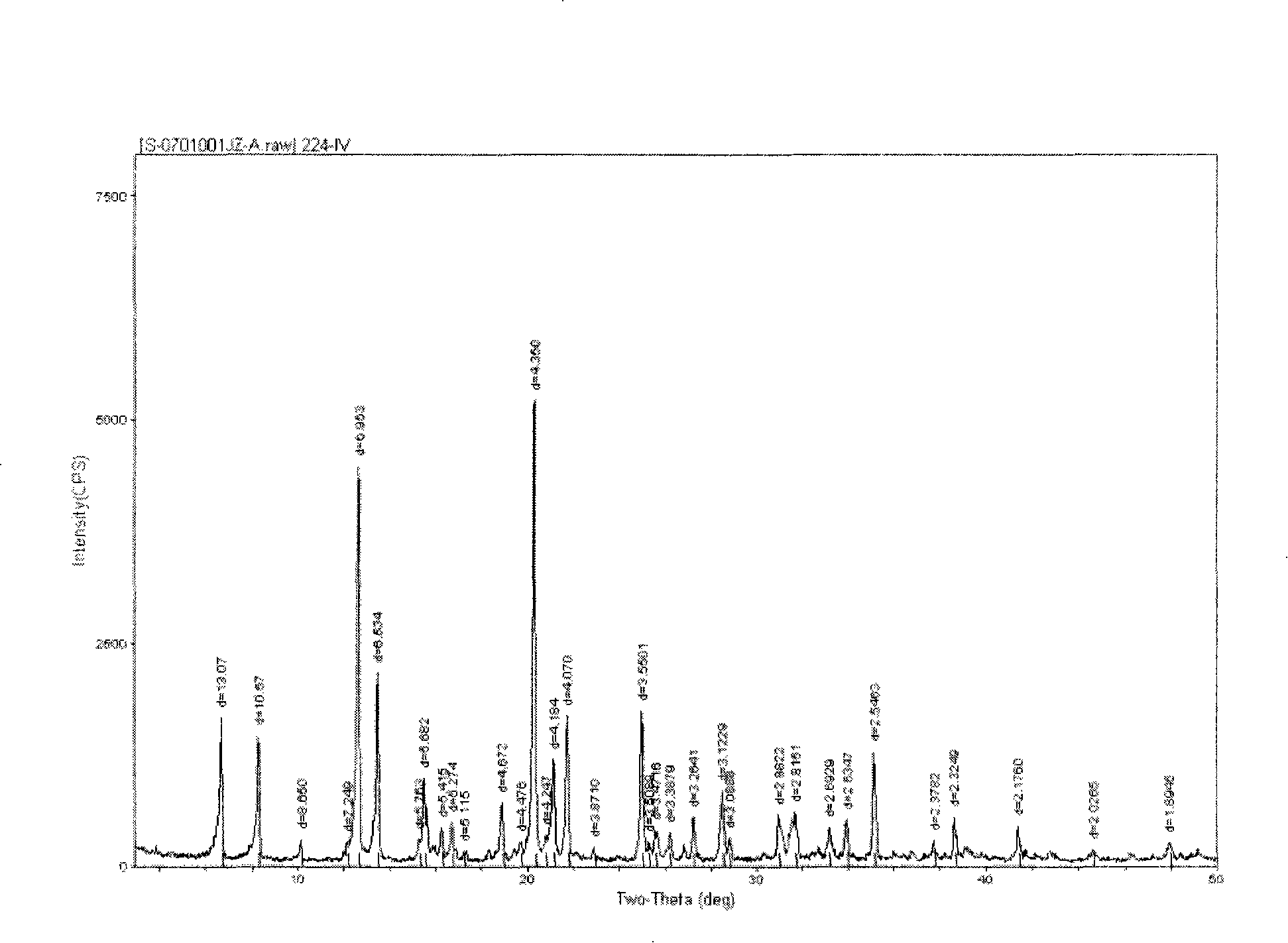
Method for preparing hydrochloric venlafaxine new crystal C
A technology of venlafaxine hydrochloride and its crystal form, which is applied in the preparation of venlafaxine hydrochloride and crystal form C of venlafaxine hydrochloride, and can solve the problems of high solvent recovery cost, low yield of crystal form C and solvent usage Achieving the effects of low cost, less solvent consumption and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of crude venlafaxine hydrochloride:
[0021] The 1-[(2-amino)-1-(4-methoxyphenyl) ethyl] cyclohexanol that 100g HPLC chromatographic purity is greater than 96% is added in the there-necked bottle of 1000ml, then adds the formaldehyde of 235g 36% and 145g 85% formic acid, heat up and reflux for 24 hours, cool down to 10°C, neutralize with 30% sodium hydroxide solution to pH 11, extract with 200ml×3 ethyl acetate three times, concentrate, and use hydrogen chloride methanol after concentration Adjust the pH value of the solution to 2-3, then concentrate the methanol, add 500ml of ethyl acetate for crystallization, cool down to 0-5°C and suction filter to obtain the crude venlafaxine hydrochloride.
[0022] Preparation of Venlafaxine Hydrochloride Form C:
[0023] At room temperature, add 1000g of isopropanol and 100g of crude venlafaxine hydrochloride to a 2000ml three-neck flask with a condenser tube and a thermometer, then raise the temperature to 60°C until...
Embodiment 2
[0025] Preparation of Venlafaxine Hydrochloride Form C:
[0026] At room temperature, add 600 g of n-butanol and 100 g of the crude venlafaxine hydrochloride prepared in Example 1 to a 1000 ml three-necked flask with a condenser tube and a thermometer, then raise the temperature to 80° C. until the system dissolves, After the dissolution, the temperature was lowered to 0°C for crystallization for 1 hour. During the crystallization process, it was stirred continuously. The pressure is -0.06MPa for suction filtration; after suction filtration, the filter cake is washed with 50g n-butanol for 1 to 5 times to continue suction filtration, and the filtrate after draining is recycled, and the filter cake is placed in a vacuum oven (model: DZF-6020, (manufactured by Shanghai Boxun Industrial Co., Ltd. Medical Equipment Factory), dried for 2-3 hours at a temperature of 60-80°C and a vacuum of 0.08Mp to 0.1Mp to obtain 92.4g of the new crystal form C of venlafax hydrochloride. rate gre...
Embodiment 3
[0028] Preparation of Venlafaxine Hydrochloride Form C:
[0029] At room temperature, in the there-necked flask that 1000ml has condenser tube and thermometer, add the mixed solvent of 400g ethyl acetate: methyl alcohol=4: 1 (weight ratio), the venlafaxine hydrochloride crude product prepared with 100g embodiment 1, Then raise the temperature to 80°C until the system is dissolved, and then cool down to 5°C for crystallization for 1.5 hours. Stir continuously during the crystallization process, and then pass the SHZ-DC(III) circulating water vacuum pump after the crystallization (Manufactured by Yingyu Yuhua Instrument Factory in Gongyi City) Control the negative pressure to be -0.06MPa and carry out suction filtration; after suction filtration, the filter cake is washed 1 to 5 times with a mixed solvent of 20g ethyl acetate:methanol=4:1 (weight ratio) Continue suction filtration, recycle the filtrate after drying, put the filter cake in a vacuum oven (model: DZF-6020, manufact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com