Application of estradiol benzoate or pharmaceutically acceptable salt thereof in preparation of anti-coronavirus drugs
A technology of estradiol benzoate and coronavirus, applied in the field of medicine, can solve problems such as no estradiol benzoate yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
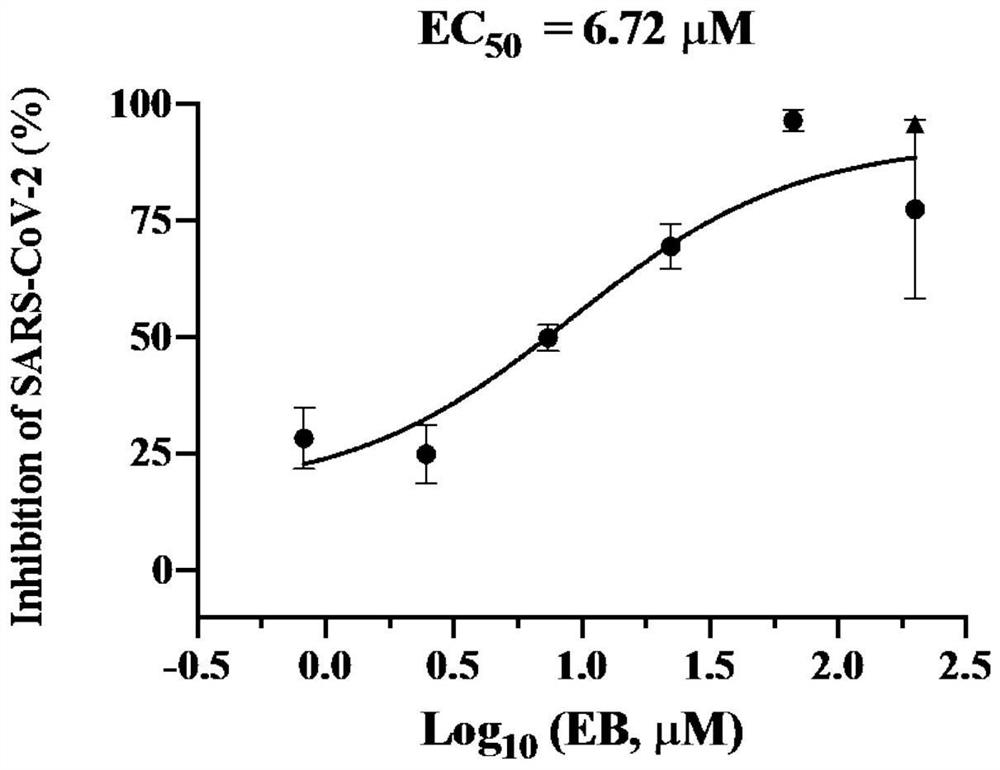
[0034] Example 1 Inhibitory activity detection of estradiol benzoate to SARS-CoV-2 in vitro
[0035] 1. Drug inhibitory activity test:
[0036] 1) Take Vero-E6 cells in the logarithmic growth phase, 3*10^5 cells / well, inoculate in a 48-well plate, 37°C, 5% CO 2 Incubate overnight.
[0037]2) Drug pre-incubation: the drug is diluted with DMEM medium containing a total volume of 2% fetal bovine serum. The initial concentration of the drug is set to 200 μM (the solvent is DMSO), the drug is diluted threefold, and 3 replicate holes are set for each concentration, with a total of 6 drug gradients (200, 66.67, 22.22, 7.41, 2.47, 0.82 μM); set the solvent Dimethyl sulfoxide (DMSO) was used as a control group, which was diluted with DMEM medium containing 2% fetal bovine serum in total volume, and given the same volume of DMSO as the drug. After removal of the cell supernatant, 100 μl of diluted drug was added to each well of the 48-well plate in 1) for the experimental group, and ...
Embodiment 2
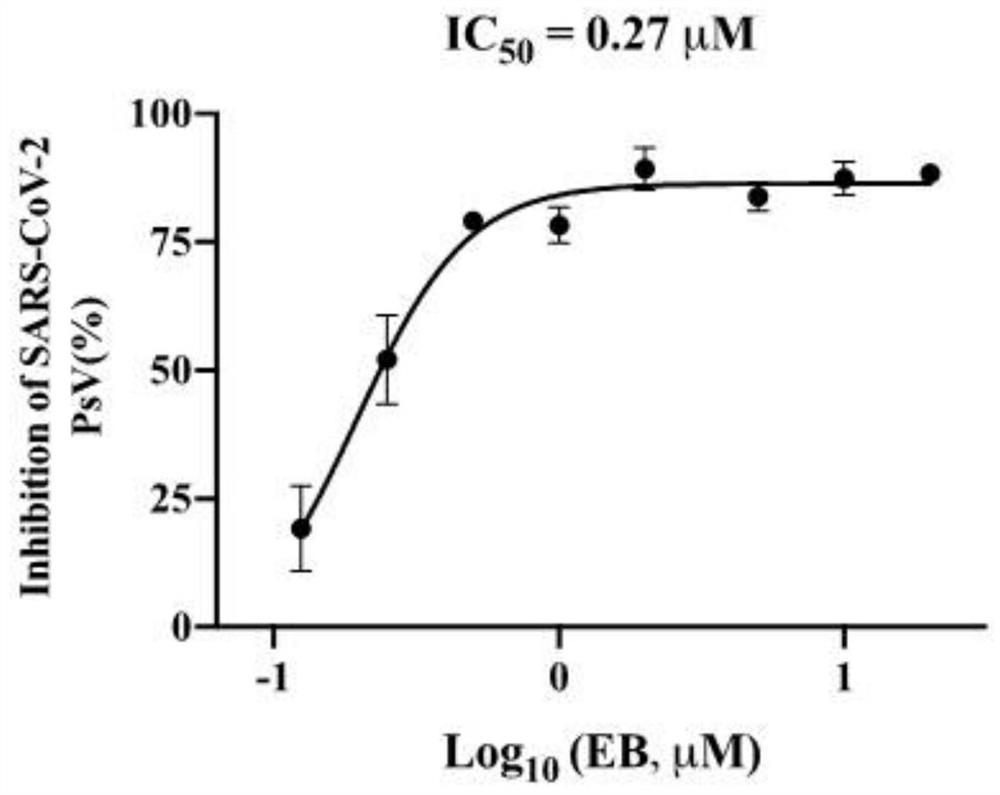
[0064] Example 2 Estradiol Benzoate Inhibitory Activity Detection of SARS-CoV-2S Pseudovirus Entry
[0065] 1. Method:
[0066] 1) SARS-CoV-2S pseudovirus packaging:
[0067] 4*10^5 HEK-293T cells in the logarithmic growth phase / ml, 2ml per well were uniformly seeded in a 6-well plate. 37°C, 5% CO 2 Incubate for 24 hours in a cell culture incubator. Replace the fresh medium half an hour before transfection, and use 100 μl blank DMEM medium to prepare plasmid dilution and transfection reagent (PolyJet) dilution respectively. The preparation ratio for each well is as follows (plasmid DNA needs to be extracted with an endotoxin-free extraction kit) :
[0068] pNL4-3.Luc.R-E-1000ng
[0069] pcDNA3.1-SARS-CoV-2-S 500ng
[0070] PolyJet 6μl
[0071] The specific preparation method is as follows: pNL4-3.Luc.R-E-plasmid and pcDNA3.1-SARS-CoV-2-Spike plasmid are simultaneously added to 100 μl blank DMEM medium and mixed, and PolyJet is diluted and mixed with 100 μl blank DMEM me...
Embodiment 3
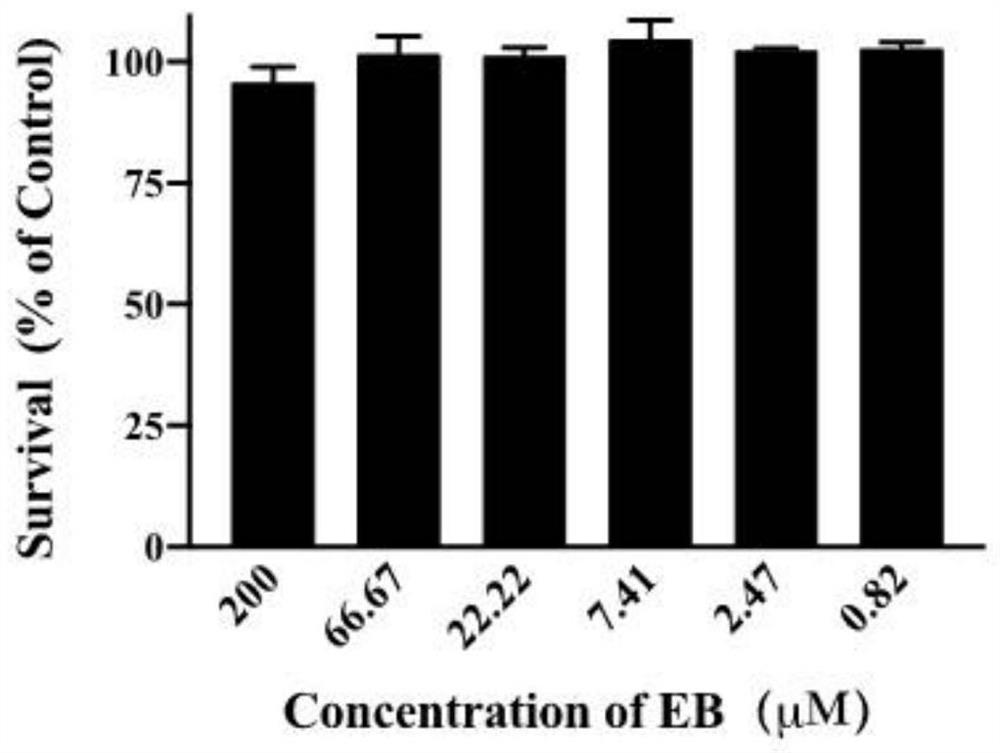
[0077] Cytotoxicity detection of embodiment 3 estradiol benzoate
[0078] 1. Method:
[0079] 1) Cell inoculation:
[0080] For Vero-E6 and 293T-ACE2 cells in the logarithmic growth phase, adjust the cell density to 1*10^4 cells / well, inoculate 100 μL / well in 96-well plates, and culture overnight.
[0081] 2) Drug concentration design:
[0082] Vero-E6 cells: 6 concentration gradients were diluted 3 times in DMEM medium containing a total volume of 2% fetal calf serum before administration, and the initial concentration of the drug was set to 200 μM (200, 66.66, 22.22, 7.41, 2.47, 0.82 μM ); Add 100 μL of the diluted drug in each well to the Vero-E6 cells in the 96-well plate in 1), with a final volume of 200 μL in each well. Three replicate wells were set up for each drug concentration. The DMSO solvent treatment group was used as the blank control.
[0083]293T-ACE2 cells: 8 concentration gradients were diluted 2-fold in DMEM medium containing 2% fetal bovine serum in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com