PH-sensitive double-drug skeleton polymer prodrug and preparation method and application thereof
A polymer and dual-drug technology, applied in drug combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve problems such as difficult to achieve precise drug delivery, and achieve the effects of completely inhibiting tumor growth, enhancing retention, and accelerating drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A pH-sensitive double-drug backbone polymer prodrug, the structure of which is shown in formula VI:
[0073]
Embodiment 2
[0075] Preparation of pH-sensitive dual-drug backbone polymer prodrugs:
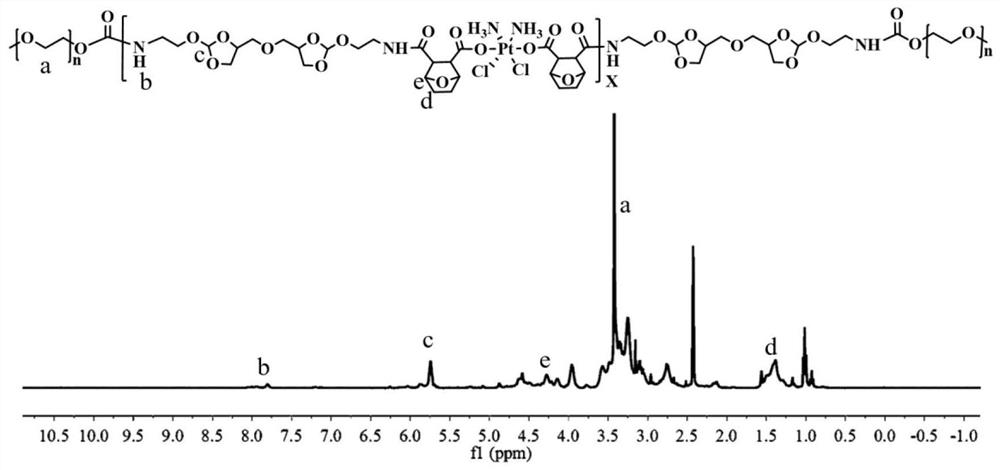
[0076] The synthetic route of the pH-sensitive dual-drug backbone polymer prodrug is as follows:
[0077]
[0078] The preparation steps of the pH-sensitive double-drug backbone polymer prodrug are as follows:
[0079] Formula I synthetic steps:
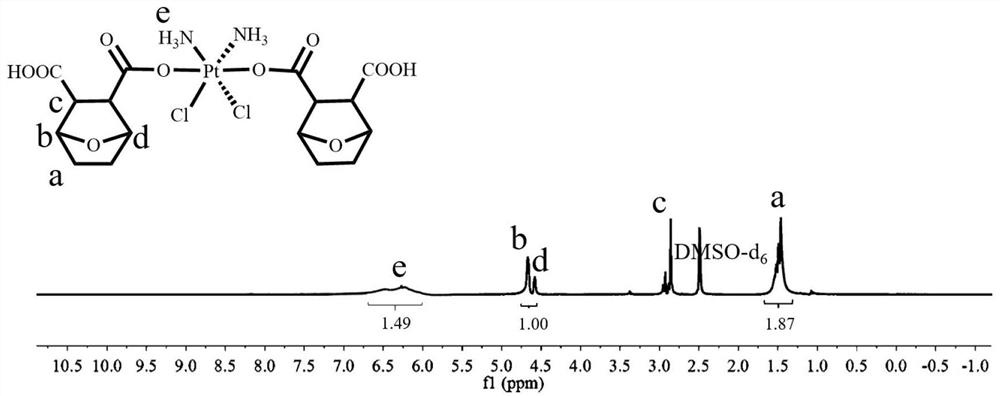
[0080] H 2 o 2 Dihydroxycisplatin (500mg, 1.493mmol), norcantharidin (1004mg, 5.972mmol) and triethylamine (catalytic amount) obtained by oxidation of the aqueous solution were dissolved in DMF, stirred at 65°C for 24 hours, and the solvent was distilled off under reduced pressure . Finally, the cisplatin-norcantharidin conjugate of formula I was obtained by precipitating twice with glacial ether. Formula I 1 H NMR picture as figure 1 shown.
[0081] Formula III synthesis steps:
[0082] Under the protection of nitrogen, polyethylene glycol monomethyl ether 550 (550mg, 1mmol) and CDI (648mg, 4mmol) were dissolved in anhydrous dichloromethane (10mL) ...
Embodiment 3
[0087] Preparation, particle size and electron microscope observation of pH-sensitive dual-drug backbone polymer prodrug nanoparticles:
[0088] Take 30 mg of the polymer prodrug in Example 2 and add it into 1 mL of DMSO to fully dissolve it, then add it dropwise into phosphate buffer (20 mL, pH 7.4, 0.01 M), and stir for 2 h. Then, the mixture was dialyzed against deionized water (MWCO 3500Da) for 24h, and the nano-prodrug particles were obtained after lyophilization.
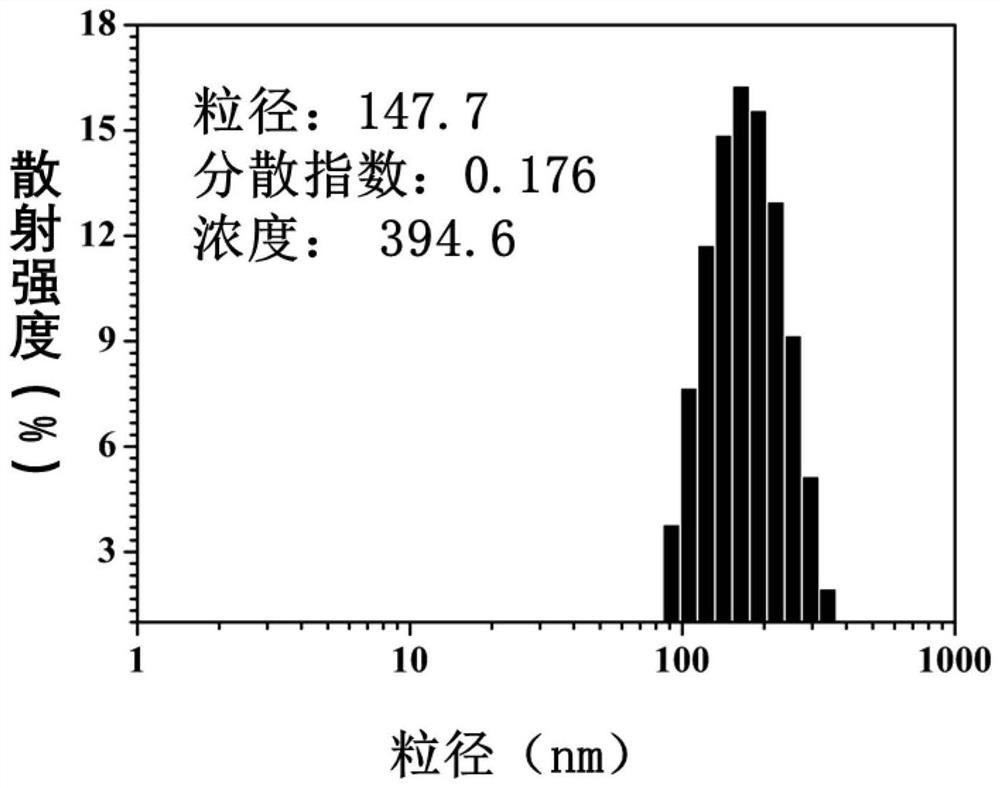
[0089] Disperse the polymer prodrug nanoparticles in 0.01M pH 8.0 phosphate buffer, take 1mL of the nanoparticle solution, dilute it to an appropriate concentration, and measure its particle size with DLS (Malvern, Zeta-sizer Nano-ZS90) at room temperature and distribution, particle size and distribution such as image 3 As shown, it can be seen that the size of the prepared polymer prodrug nanoparticles is about 150nm, and the dispersion is good.
[0090] Take a drop of diluted polymer prodrug nanoparticle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com