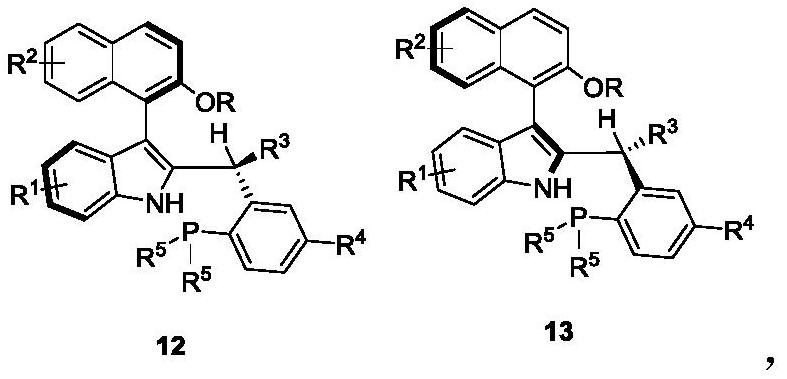
Axial chiral naphthalene-indole phosphine catalyst as well as preparation method and application thereof
A technology of indoles and catalysts, applied in the field of axial chiral naphthalene-indole phosphine catalysts and their preparation, and axial chiral biaryl phosphine compounds, can solve the problems of high synthesis cost, achieve large steric hindrance, reduce Cost, the effect of enhancing the catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
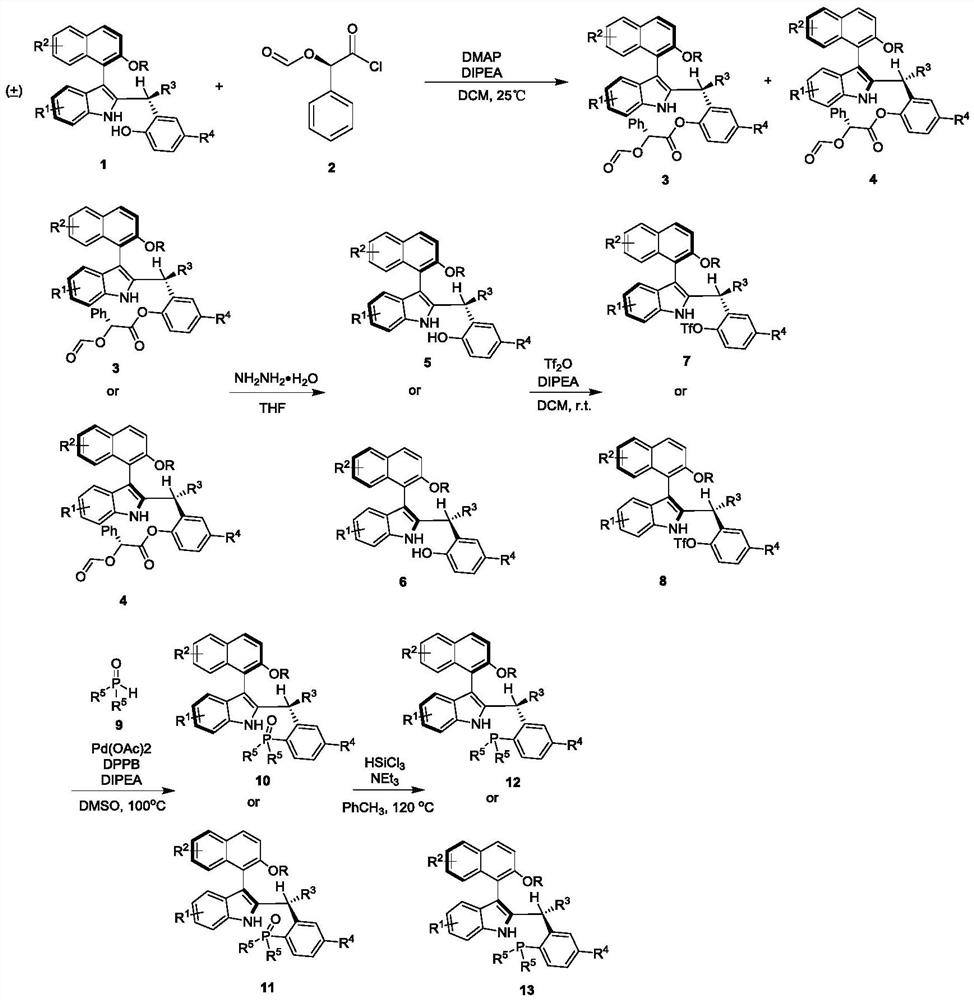
[0041] Prepare the axial chiral indole-naphthalene phosphine catalyst as follows:
[0042]
[0043] Prepare the axial chiral indole-naphthalene phosphine catalyst as follows:
[0044] (1) Add 0.5 mmol of formula 1 compound, 0.05 mmol of 4-dimethylaminopyridine, 0.6 mmol of formula 2 compound, 1 mmol of diisopropylethylamine in 5 milliliters of anhydrous dichloromethane, stir at room temperature for 12 Hour. TLC traced the reaction to the end, added 2mL of 1N hydrochloric acid to quench, and then extracted 3 times with 15mL of ethyl acetate, combined the organic phases and dried them with a sufficient amount of anhydrous sodium sulfate, filtered, concentrated, and separated by silica gel column chromatography Purify to obtain compounds of formula 3 and formula 4.
[0045] The structural characterization data of the compound of formula 3 are as follows:
[0046] yield:35%(116.0mg); dr>95:5; white solid; m.p.92.2-92.7℃; [α] D 20 =+32.9(c 0.47, Acetone); 1 H NMR (400MHz, ...
Embodiment 11
[0068]
[0069] Add 0.1 mmol o-methylene quinone compound 14, 0.2 mmol MBH ester compound 15, and 0.02 mmol axial chiral indole-naphthalene phosphine catalyst 13a to 1 ml of chloroform, and react at 35°C for 12 hours. After the reaction was tracked by TLC to the end, after washing and concentration, the compound of formula 16 was obtained by separation and purification through silica gel column chromatography.
[0070] The structural characterization data of the compound of formula 16 are as follows:
[0071] Yield: 92% (39.2mg); colorless oil; 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.44–7.27(m,9H),7.26–7.16(m,1H),6.58(s,1H),6.48(s,1H),6.43–6.36(m,2H),6.33–6.23 (m,1H),5.94(s,1H),5.91(d,J=1.6Hz,2H),5.34(d,J=6.0Hz,1H),5.22(s,2H),4.06–3.84(m, 1H); 13 C NMR (100MHz, CDCl 3)δ (ppm): 165.2, 153.7, 147.9, 142.0, 139.1, 136.7, 135.4, 131.4, 129.8, 128.6, 128.5, 128.3, 127.5, 126.4, 126.1, 119.1, 105.4, 101.3, 93.0, 87.0, IR(KBr):3488,3027,2893,1721,1473,1276,1261,1143,1038,826,750,6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com