Synthetic liver-tropic adeno-associated virus capsids and uses thereof
A capsid and capsidization technology, applied in the field of transducing human primary hepatocytes and cell lines, can solve the problem of insufficient liver targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0210] Construction of AAV capsid gene shuffling library
[0211]The construction of the AAV capsid gene shuffling library was as previously described (Yang et al., Proc.Natl.Acad.Sci.USA106(10):3946(2009); Yang et al., Meth.Mol.Biol.709:127 (2011)), the capsid genes of AAV serotypes 1, 2, 3B, 4, 6, 7, 8 and 9 were cloned into the same vector pXX-UF1 containing the AAV2 inverted terminal repeat (ITR) and AAV2 Rep gene -AAV. These plasmids containing ITR, AAV2 Rep and each of the eight different Cap genes were named pXX-UF1-AAVn in sequence. These capsid genes were amplified by primers CAP-5'5'-CCC-AAGCTTCGATCAACTACGCAGACAGGTACCAA-3' (SEQ ID NO:7) and CAP-3'5'-ATAAGAAT-GCGGCCGC-AGAGACCAAAGTTCAACTGAAACGA-3' (SEQ ID NO:8). and mixed in the same ratio for DNA shuffling (Stemmer, Proc. Natl. Acad. Sci. USA 91:10747 (1994)). Briefly, 4 μg of DNA template was briefly treated with 0.04 UDNase I at 15°C, and 300–500 and 500–1000 bp fragments were purified from agarose gels using pfu...
example 2
[0213] In vivo enrichment in mouse liver
[0214] For in vivo screening of AAV variants targeting the liver, by 1x10 11 vg (vector genome) / mouse The AAV chimeric library was administered to adult C57BL / 6J mice via the tail vein. After two weeks, mouse tissues, including liver and other tissues, were collected for genomic DNA isolation using phenol / chloroform extraction. After PCR amplification of total liver DNA using universal primers for capsid genes, the PCR products were recloned into an AAV plasmid backbone containing the AAV2 ITR and Rep genes. Therefore, both the capsid gene PCR product and the pXX-UF1-AAV vector were digested with HindIII / NotI and ligated for cloning. A second round of infectious AAV particles was generated from the first round of in vivo enrichment and reinjected into mice. This in vivo enrichment process was repeated three times in mice.
[0215] The final PCR product highly enriched for capsid genes was cloned into a universal precursor of an AA...
example 3
[0217] Generation of AAV vectors containing reporter genes
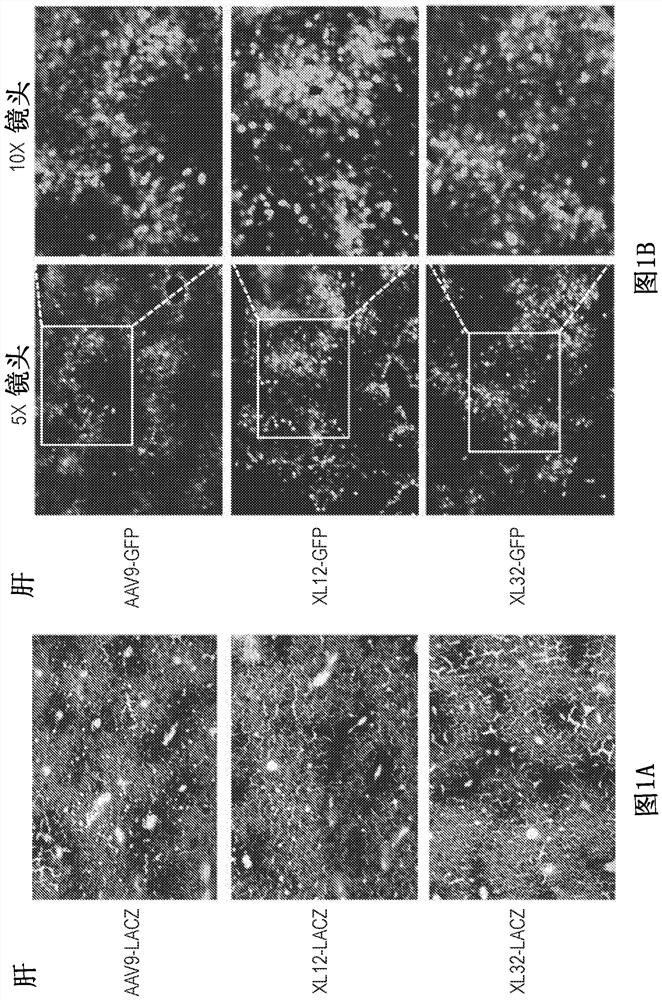
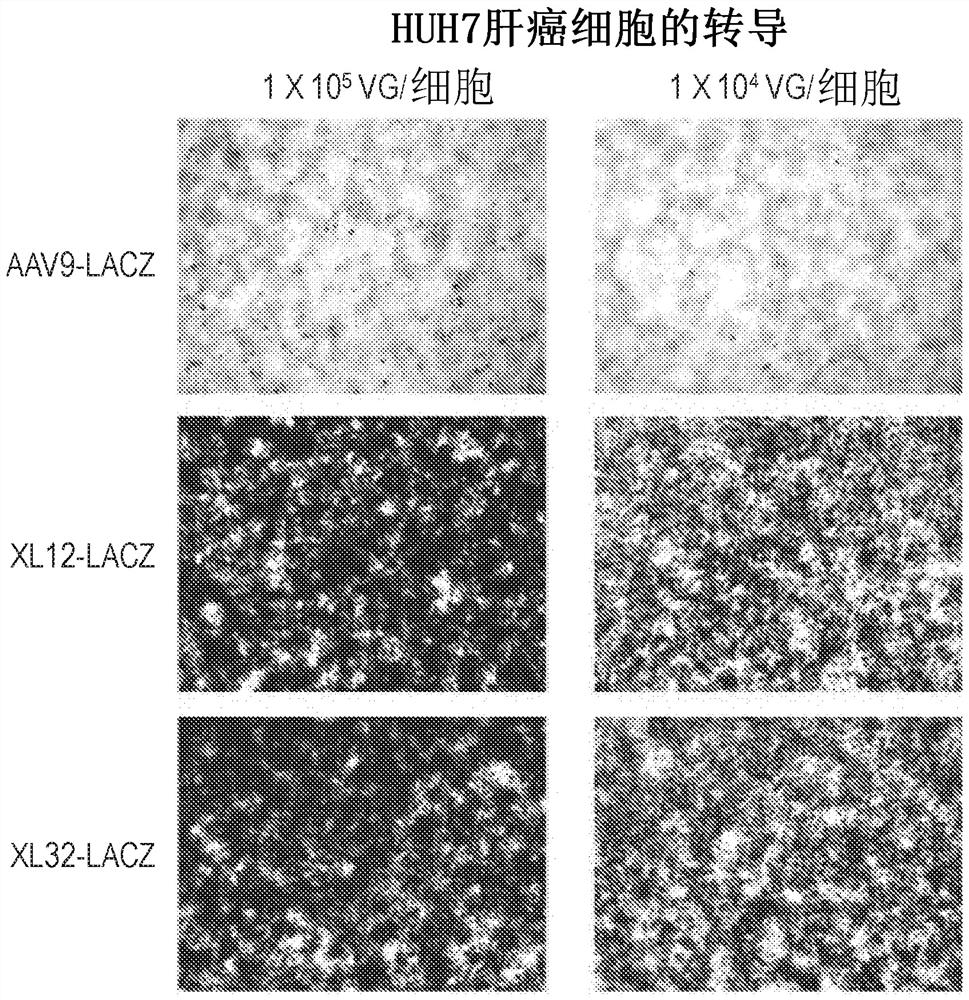
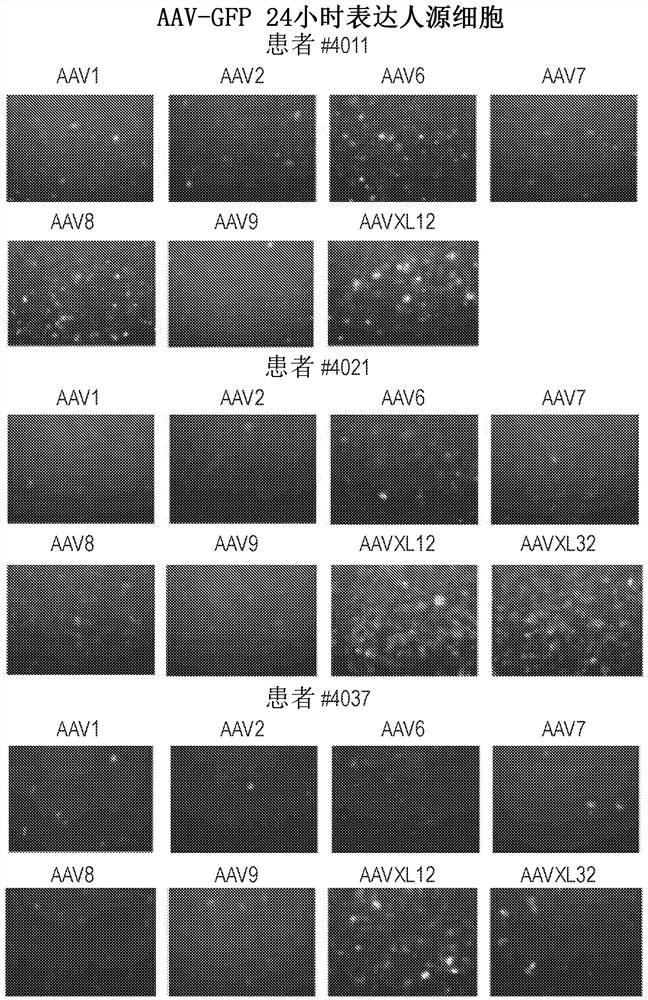
[0218] Capsid-specific packaging plasmids were prepared by conventional large-scale preparation methods and purified by CsCl density ultracentrifugation twice. These plasmids are used to package reporter genes such as green fluorescent protein GFP and β-galactosidase under the transcriptional control of the ubiquitous promoter CMV or CB (CMV enhancer plus chicken β-actin basal promoter). Lac-Z gene) AAV vector. AAV vectors were packaged in each new capsid and purified by conventional double CsCl density ultracentrifugation. The titer of the reporter vector was determined relative to the known copy number of the corresponding reporter vector plasmid DNA by DNA dot blot. All vector yields were within the normal range.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com