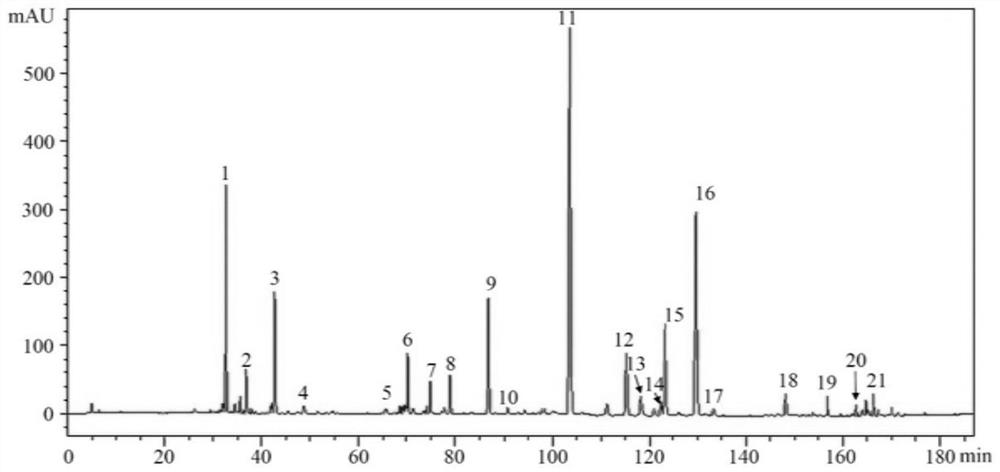
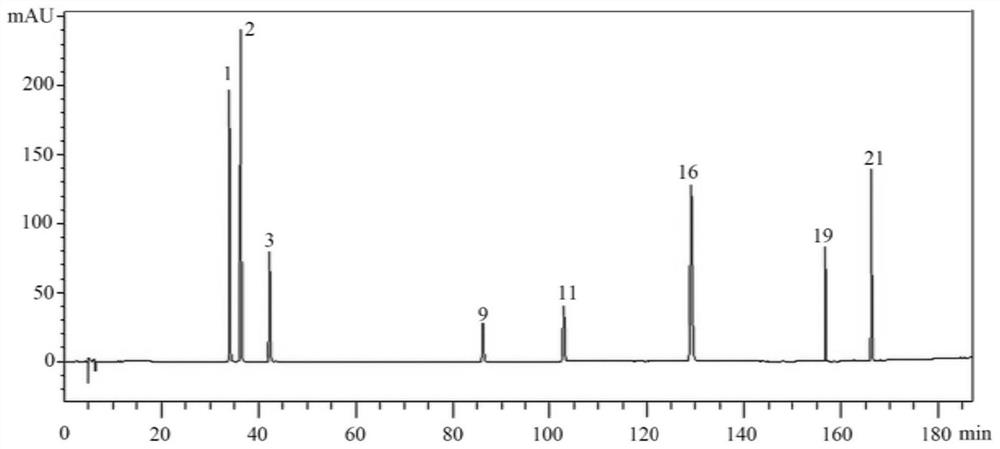
HPLC quality control construction method and application of lung-heat clearing decoction preparation
A construction method and technology of Qingfei Decoction, which is applied in the quality control direction of commercially available Qingfei Decoction preparations, and the construction of HPLC quality control of Qingfei Decoction preparations, can solve the problems recorded in Qingfei Decoction, difficult separation of components with similar polarities, There are many problems such as the smell of medicine, to achieve the effect of promoting inheritance and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Qingfei decoction quality control construction method
[0032] In this embodiment, the Qingfei Decoction quality control construction method adopts the following steps:
[0033] 1. Extraction and processing of related preparations of Qingfei Decoction;
[0034] 2. Preparation of a mixed reference solution of 8 components including chlorogenic acid, amygdalin, geniposide, hesperidin, baicalin, wogonoside, ammonium glycyrrhizinate, and schisandrin A;
[0035] 3. Methodological investigation of high performance liquid chromatography content determination of 8 components in step 2, and methodological investigation of fingerprints.
[0036] That is, the quality control construction method in this embodiment covers two aspects: the determination of the content of active ingredients and the construction of fingerprints. The process of determining the content and the construction of fingerprints are basically the same, and will be described in a unified manner bel...
Embodiment 2
[0083] Example 2 Application of HPLC quality control construction method in the direction of quality control of commercially available Qingfei Decoction preparations
[0084] 1. Information on commercially available preparations of Qingfei Decoction:
[0085] Qingfei decoction concentrated tablets (manufacturer: Hong Kong Xianglan Pharmaceutical Co., Ltd., batch number: G26419), hereinafter referred to as preparation A; Qingfei decoction concentrated granules (manufacturer: Hong Kong Xianglan Pharmaceutical Co., Ltd., batch number: K09916), hereinafter Referred to as preparation B; Qingfei decoction extract granules (manufacturer: Japan Tsumura Pharmaceutical Co., Ltd., batch number: N34151), hereinafter referred to as preparation C; 18102431), hereinafter referred to as preparation D; Qingfei decoction concentrated powder (manufacturer: China Taiwan Xianfeng Co., Ltd., batch number: E073RR1), hereinafter referred to as preparation E, Qingfei decoction commercially available p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com