Antibody composition and application thereof in screening of post-transplant lymphoproliferative disorders (PTLD)
A technology of lymphocyte proliferation and antibody composition, applied in the biological field, can solve the problems of no FCM detection of peripheral blood early screening means, no standardized plan, and severe symptoms of PTLD patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 reagent
[0063] The antibody combinations used in this example are:
[0064]A1 component is: CD19-Pe-Cy7, CD38-APC, CD20-APC-Cy7, CD56 BV421, CD45 V500, the above five monoclonal antibody reagents are mixed according to volume ratio 3:3:2:3:3 in the first container;
[0065] A2 component is: cytoplasmic kappa-FITC, cytoplasmic lambda-PE, and the two monoclonal antibodies are packed in the second container according to the volume ratio of 2:2;
[0066] The antibodies in this example are commercially available, among which, cytoplasmic kappa-FITC and cytoplasmic lambda-PE are products of Dako Company of Denmark, and other fluorescein directly labeled antibodies are products of Becton Dickinson Company of the United States.
[0067] Optionally add red blood cell lysate and put it in the third container, split the membrane breaking agent A and B liquid into the fourth container and the fifth container, put PBS buffer in the sixth container...
Embodiment 2
[0068] Example 2 Processing of Specimen
[0069] According to the results of cell counting, add heparin or EDTA anticoagulated peripheral blood samples into the flow tube, and ensure that the amount of cells added is about 2×10 6 (1×10 6 -1×10 7 ), with a volume not exceeding 160 μl; first add 3ml PBS and incubate at 37°C for 5 minutes, centrifuge at 300-450g for 5 minutes to remove the supernatant, repeat the incubation and centrifugation once, then add 3ml PBS to wash once (mix well, centrifuge at 300-450g for 5 minutes, Remove the supernatant), then add 14 μl of five membrane monoclonal antibody reagents labeled with different fluorescein (ie, component A1 of Example 1) to the flow tube according to Table 1, mix well with the cell suspension and keep away from room temperature Incubate in light for 15 minutes, add 100 μl membrane disrupting agent A solution, incubate at room temperature in the dark for 5 minutes, add 3ml 1× hemolysin, incubate in the dark for 10 minutes t...
Embodiment 3
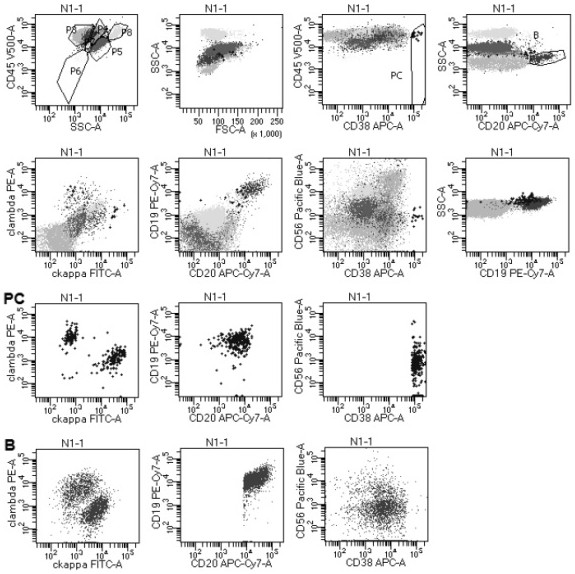
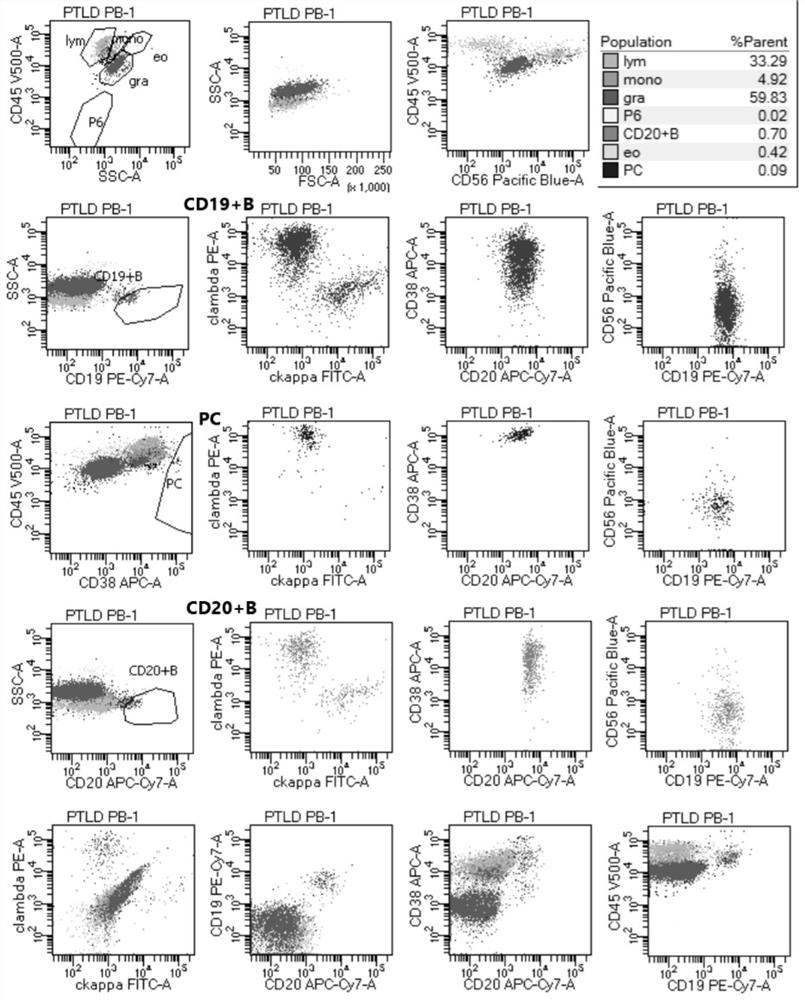
[0072] Example 3 Detection of Specimen
[0073] The specimens processed according to the method of Example 2 were tested on a 3-laser 8-color FACS Canto II flow cytometer from Becton Dickinson Company in the United States. After obtaining 500,000 cells per tube, the data was analyzed using diva 2.8 software. Among them, CD45 weak expression (dim) / CD38 strong expression (bright, bri) were selected to select plasma cells, and CD19 / side scatter (side scatter, SSC) and CD20 / SSC antibodies were selected to select B cells .
[0074] The evaluation criteria for monoclonal B cells and plasma cells involved in FCM screening for PTLD are as follows:
[0075] (1) Simultaneous B cell validation using CD19 / SSC and CD20 / SSC.
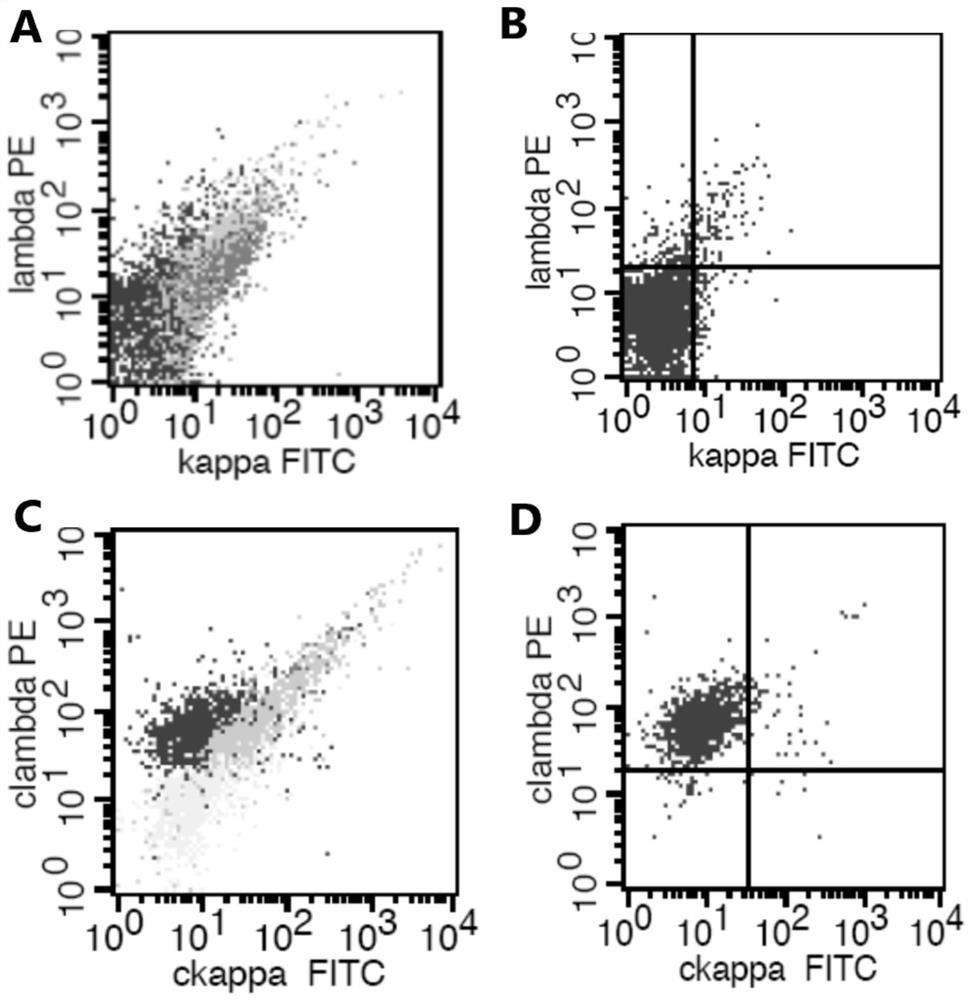
[0076] (2) Judgment of monoclonal B cells: Under normal circumstances, all B cells of CD19 / SSC and CD20 / SSC, and any part of B cells with different expression intensities of CD19 and CD20, should be polyclonal cells, that is, cytoplasmic kappa / Cytoplasmic lambda i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| cover factor | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com