Induced T-to-natural killer feeder cell as well as preparation method and application thereof
A feeder cell, NK cell technology, applied in the biological field, to achieve the effect of strong tumor killing ability and stable killing function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Construction of reprogrammed NK cells
[0047] (1) Design sgRNA (SEQ ID NO:6:gaccatgaactgctcacttg) according to the Bcl11b gene, and add restriction enzyme sites EcoRI and SalI at the 5' end and 3' end of the sequence after artificial synthesis;
[0048] The synthesized sequence fragment was digested with EcoRI and SalI, then connected to the PX458-gBCL11b vector containing the T7 promoter, and the recombinant plasmid was successfully constructed by sequencing.
[0049] (2) will 1×10 7 T cells were resuspended in 3 mL of T cell medium, seeded in one well of a 6-well plate, and activated by adding a combination of 100 ng / mL anti-human CD3 antibody and 100 ng / mL anti-human CD28 antibody;
[0050] After 3 days, remove the suspended cells for counting, centrifuge at 300×g for 10 min, resuspend the cell pellet in 10 mL of Opti-MEM, centrifuge at 300×g for 10 min, resuspend the cell pellet in 100 μL of Opti-MEM, add CRISPR at a concentration of 40 μM / Cas9 plasmid...
Embodiment 2
[0052] Example 2 Construction of reprogrammed NK feeder cells
[0053] (1) Artificially synthesized T2A-linked nucleic acid molecules containing genes encoding IL-12 and CD8α transmembrane regions, CD19, CD64 and CD86, and added EcoRI and BamHI restriction sites and their protective bases at both ends;
[0054] Use restriction endonucleases EcoRI and BamHI to perform double digestion on nucleic acid molecules, incubate in a water bath at 37°C for 30 minutes, and use 1.5% agarose gel electrophoresis to recover the digested products containing sticky ends;
[0055] The digested product was ligated into the linearized pLVX-EF1-MCS plasmid (containing cohesive ends) digested with EcoRI and BamHI, and the ligation system was shown in Table 1 to obtain a lentiviral vector.
[0056] Table 1
[0057] components Dosage (μL) pLVX-EF1-MCS plasmid 2(50ng) IL-12-CD19-CD64-CD86 nucleic acid molecule 10(150ng) T4 DNA Ligation Buffer 2 T4 DNA Ligase (NEB)...
Embodiment 3
[0061] Example 3 In vitro expansion of reprogrammed NK cells
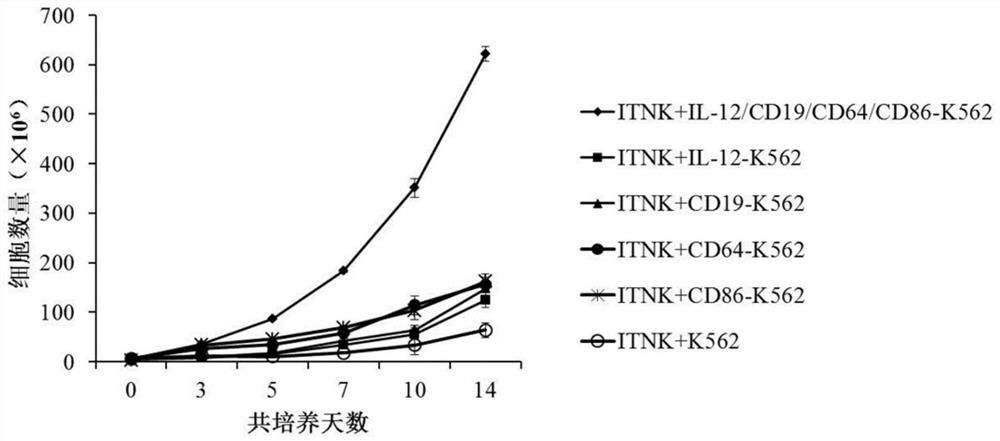
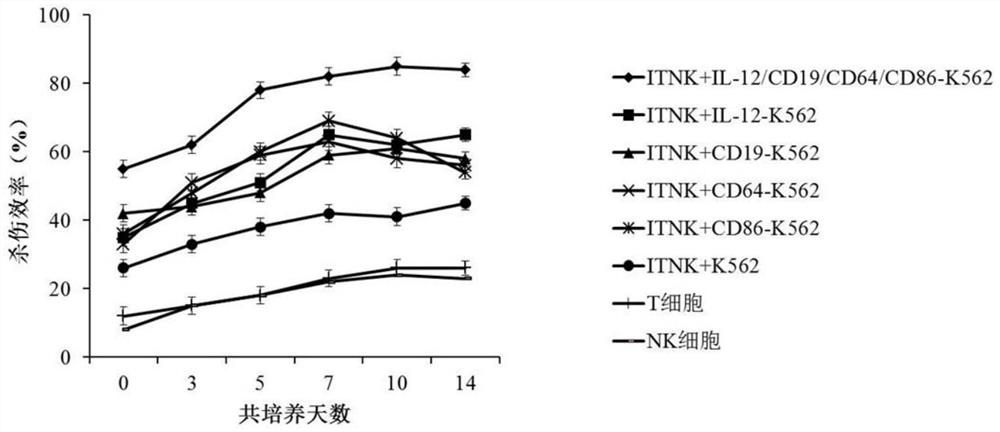
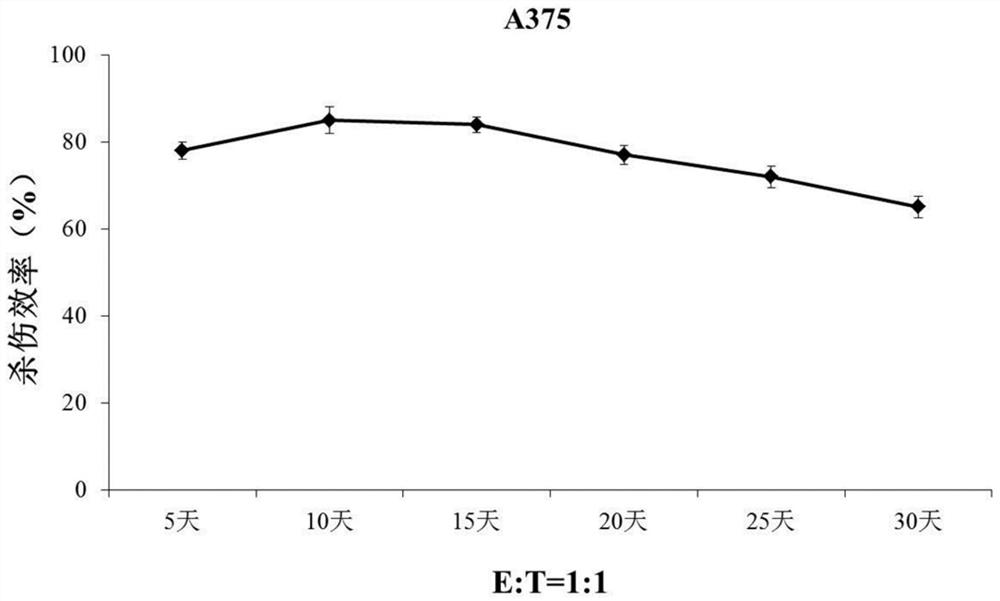
[0062] In this example, the reprogrammed NK cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, and the cells were counted after 24 hours of culture, and the cell concentration was adjusted to 2×10 6 cells / mL, add an equal proportion of reprogrammed NK feeder cells to the culture medium on day 0 and day 7, and add IL-2 at a concentration of 50 U / mL, at 37°C, 5% CO 2 Co-cultivation in the incubator under 500Gy irradiation conditions.
[0063] Such as figure 1 As shown, after the reprogrammed NK cells were co-cultured with IL-12-CD19-CD64-CD86 reprogrammed NK feeder cells, the number of cells increased significantly. After 2 weeks of culture, the number of reprogrammed NK cells increased by about 120 times, which was significantly higher than that of the control Group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com