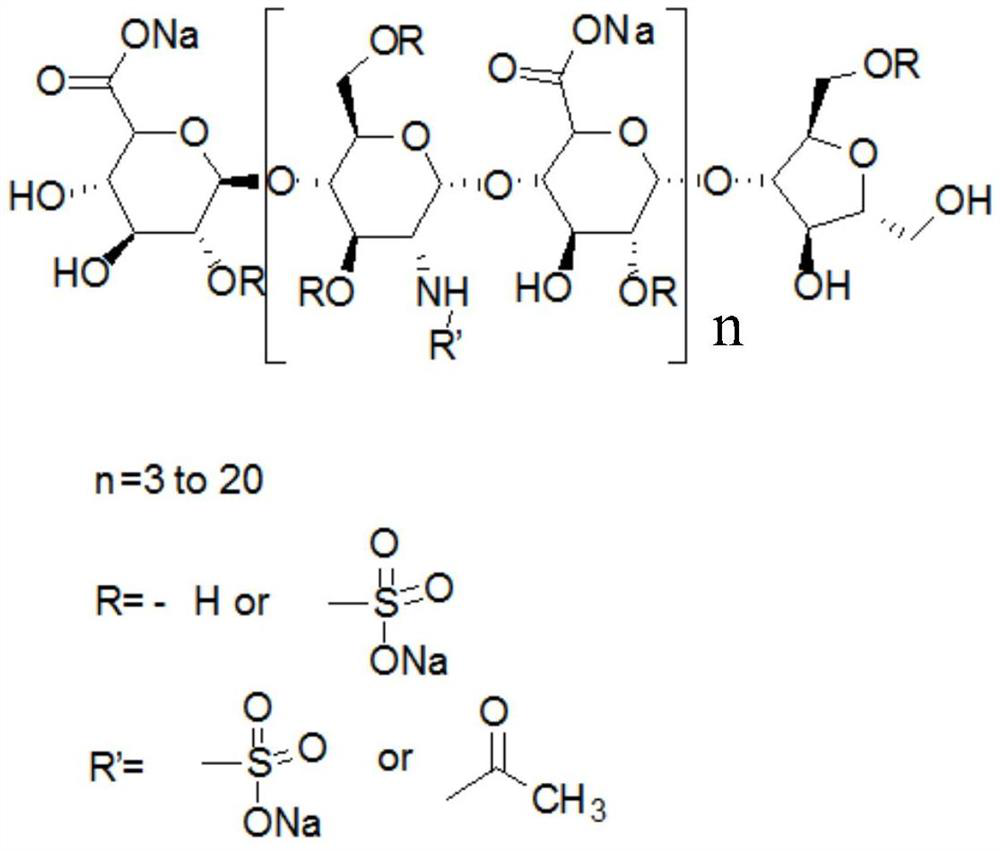
Freeze-drying process preparation method of dalteparin sodium
A technology of dalteparin sodium and dalteparin, which is applied in the field of preparing dalteparin sodium by freeze-drying, can solve the problems of excessive organic solvent residues, unqualified product properties and clarity, and unclear language, so as to improve quality and facilitate product collection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
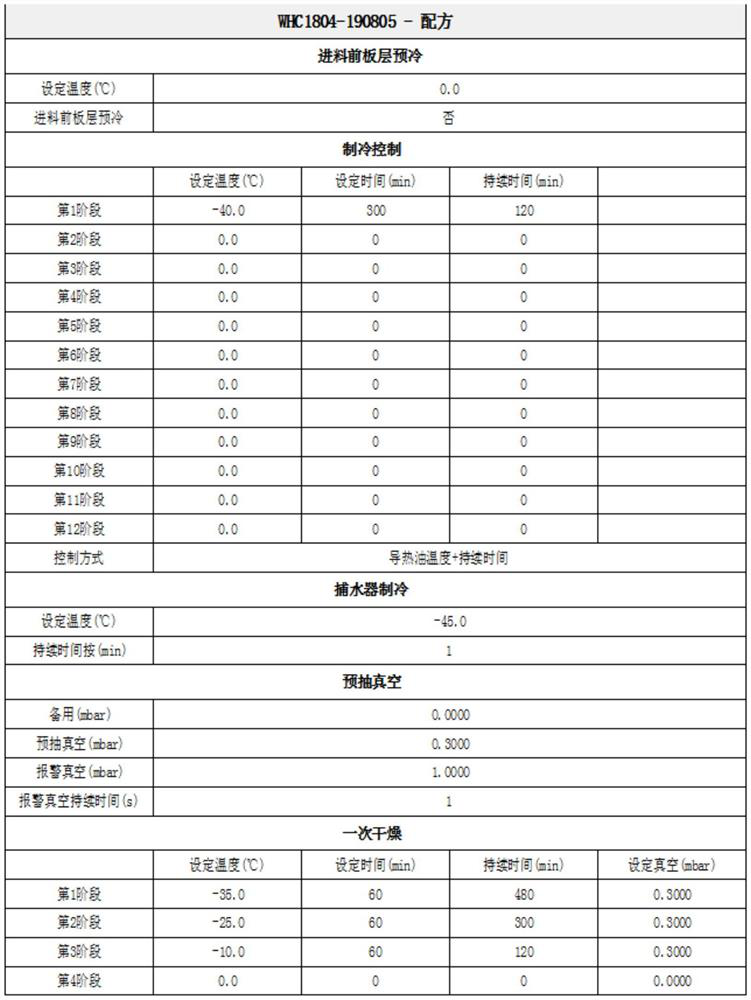
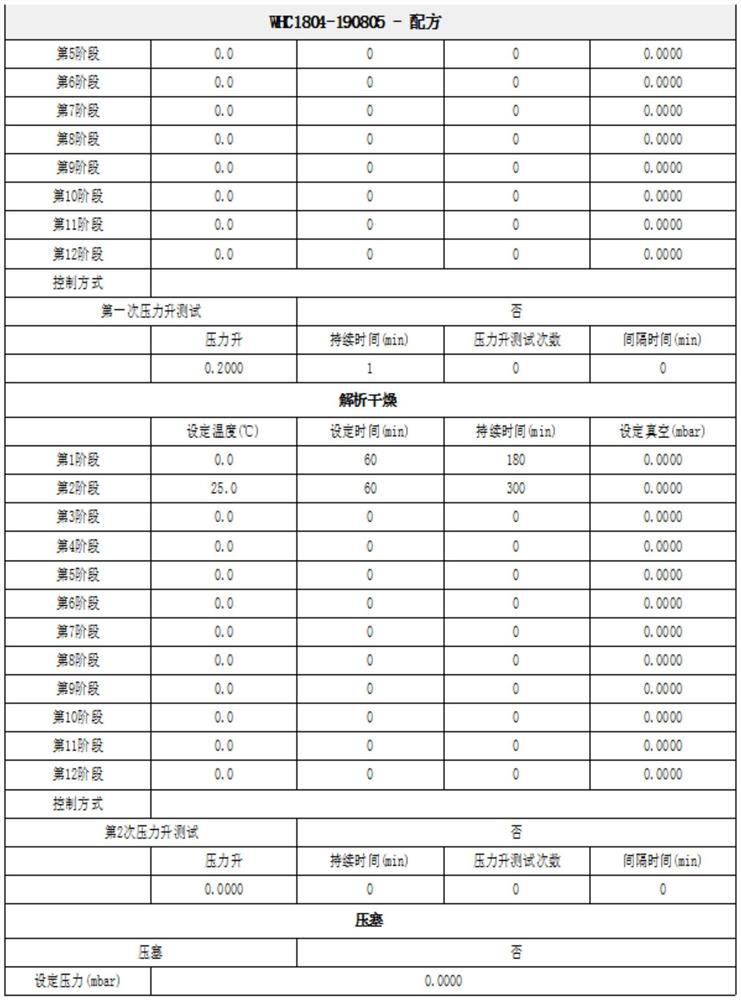
[0024] Add 1 gram of dalteparin sodium into purified water, stir, dissolve and clarify at 30°C, then continue to add water to the feed solution and adjust the volume to 10ml, filter to form a 10% dalteparin sodium aqueous solution to obtain the first dalteparin sodium aqueous solution ; transfer and place in a lyophilizer, and freeze at -40°C for 5 hours to obtain the solid matter of dalteparin sodium and water. Raise the temperature to -35°C, maintain at this temperature for 10 hours, and turn on the vacuum, set the vacuum control below 0.25mbar, raise the temperature to -25°C, maintain at this temperature for 7 hours, raise the temperature to -10°C, in This temperature was maintained for 3 hours. After the first drying, enter into analytical drying, raise the temperature to 0°C and maintain this temperature for 3 hours. Raise the temperature to 40°C, maintain at this temperature for 4-5 hours, turn off the lyophilizer, and obtain dalteparin sodium refined product A.
[002...
Embodiment 2
[0027] Add 1 gram of dalteparin sodium into purified water, stir, dissolve and clarify at 30°C, then continue to add water to the feed solution and adjust the volume to 10ml, filter to form a 10% dalteparin sodium aqueous solution to obtain the first dalteparin sodium aqueous solution ; transfer to a freeze dryer, set the freeze dryer to cool from 20°C to -40°C for 5 hours, and freeze at -40°C for 5 hours to obtain solids of dalteparin sodium and water. Raise the temperature to -35°C, maintain at this temperature for 10 hours, and turn on the vacuum, set the vacuum control below 0.25mbar, raise the temperature to -25°C, maintain at this temperature for 7 hours, raise the temperature to -10°C, in This temperature was maintained for 3 hours. After the first drying, enter into analytical drying, raise the temperature to 0°C and maintain this temperature for 3 hours. Raise the temperature to 20°C, maintain at this temperature for 4-5 hours, turn off the lyophilizer, and obtain da...
Embodiment 3
[0029] Add 1.8 g of dalteparin sodium into purified water, stir, dissolve and clarify at 30°C, continue to add water to the feed solution and adjust the volume to 10 ml, filter to form 18% dalteparin sodium aqueous solution, and obtain the first dalteparin sodium aqueous solution ; transfer and place in a lyophilizer, the lyophilizer is pre-cooled to -40°C, and frozen at -40°C for 5 hours to obtain solids of dalteparin sodium and water. Raise the temperature to -35°C, maintain at this temperature for 10 hours, and turn on the vacuum, set the vacuum control below 0.25mbar, raise the temperature to -25°C, maintain at this temperature for 7 hours, raise the temperature to -10°C, in This temperature was maintained for 3 hours. After the first drying, enter into analytical drying, raise the temperature to 0°C and maintain this temperature for 3 hours. Raise the temperature to 20°C, maintain at this temperature for 4-5 hours, turn off the lyophilizer, and obtain dalteparin sodium r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com