Preparation method of denitration and demercuration catalyst and catalyst prepared thereby
A catalyst and mercury removal technology, applied in heterogeneous catalyst chemical elements, chemical instruments and methods, physical/chemical process catalysts, etc. The effect of improving efficiency and improving sulfur resistance and water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
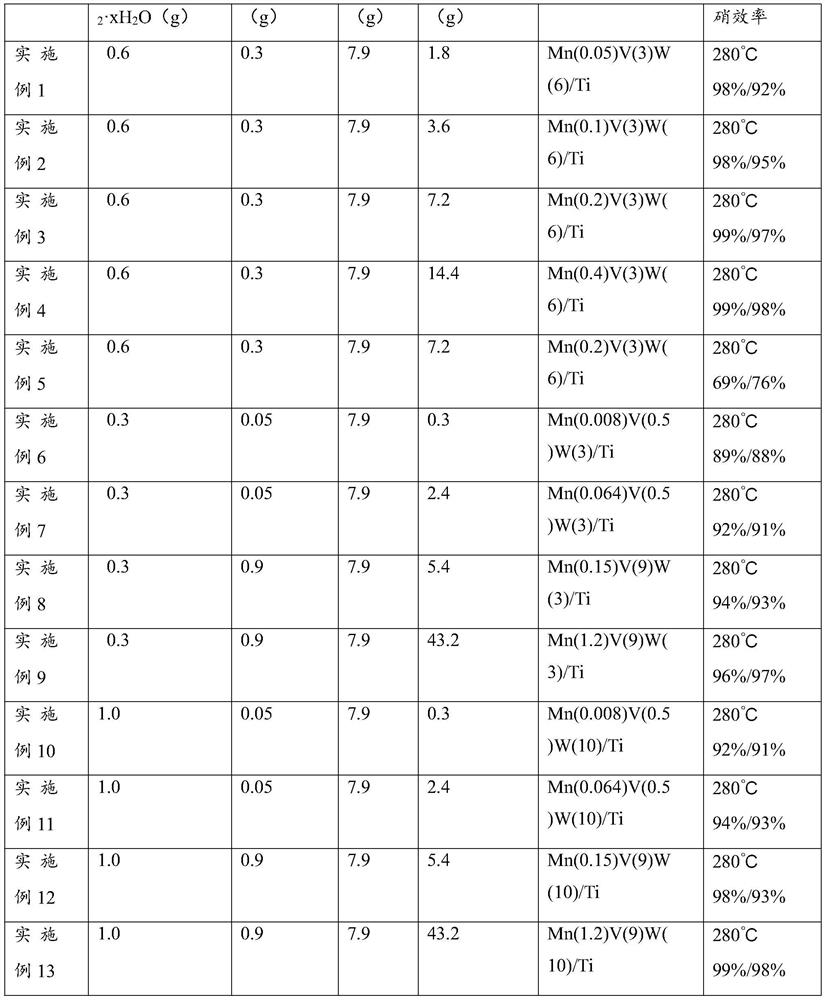
[0037] With a configured concentration of 10% HNO 3 The beaker, glass rod, magnetic rotor, etc. used to prepare the catalyst were washed with the solution and deionized water three times, and then dried; then, 100mL deionized water was poured into the dried beaker, and 0.6gH 40 N 10 o 41 W 12 ·xH 2 O, 0.3g of NH 4 VO 3 Pour the oxalic acid into the beaker, put the magnetic rotor into the beaker, place the beaker on the magnetic stirrer, heat and stir at 60°C to make it fully dissolve, the amount of oxalic acid is to ensure that the H 40 N 10 o 41 W 12 ·xH 2 O and NH 4 VO 3 Completely dissolved shall prevail; then weigh 7.9g TiO 2 (P25) powder, 1.8g Mn(NO 3 ) 2 Pour into a beaker (here H 40 N 10 o 41 W 12 , NH 4 VO 3 、TiO 2 and Mn(NO 3 ) 2 The mass ratio is equivalent to 12:6:158:36 in the claim), and continued to stir for 12 hours at normal temperature. Usually, in order to prevent the catalyst from being polluted, the mouth of the beaker can be sealed w...
Embodiment 2
[0039] With a configured concentration of 10% HNO 3 The beaker, glass rod, magnetic rotor, etc. used to prepare the catalyst were washed with the solution and deionized water three times, and then dried; then, 100mL deionized water was poured into the dried beaker, and 0.6gH 40 N 10 o 41 W 12 ·xH 2 O, 0.3g of NH 4 VO 3 Pour the oxalic acid into the beaker, put the magnetic rotor into the beaker, place the beaker on the magnetic stirrer, heat and stir at 60°C to make it fully dissolve, the amount of oxalic acid is to ensure that the H 40 N 10 o 41 W 12 ·xH 2 O and NH 4 VO 3 Completely dissolved shall prevail; then weigh 7.9g TiO 2 (P25) powder, 3.6g Mn(NO 3 ) 2 Pour into a beaker (here H 40 N 10 o 41 W 12 , NH 4 VO 3 、TiO 2 and Mn(NO 3 ) 2 The mass ratio is equivalent to 12:6:158:72 in the claim), and continued to stir for 12 hours at normal temperature. Usually, in order to prevent the catalyst from being polluted, the mouth of the beaker can be sealed w...
Embodiment 3
[0041] With a configured concentration of 10% HNO 3 The beaker, glass rod, magnetic rotor, etc. used to prepare the catalyst were washed with the solution and deionized water three times, and then dried; then, 100mL deionized water was poured into the dried beaker, and 0.6gH 40 N 10 o 41 W 12 ·xH 2 O, 0.3g of NH 4 VO 3 Pour the oxalic acid into the beaker, put the magnetic rotor into the beaker, place the beaker on the magnetic stirrer, heat and stir at 60°C to make it fully dissolve, the amount of oxalic acid is to ensure that the H 40 N 10 o 41 W 12 ·xH 2 O and NH 4 VO 3 Completely dissolved shall prevail; then weigh 7.9g TiO 2 (P25) powder, 7.2g Mn(NO 3 ) 2 Pour into a beaker (here H 40 N 10 o 41 W 12 , NH 4 VO 3 、TiO 2 and Mn(NO 3 ) 2 The mass ratio is equivalent to 12:6:158:144 in the claim), and continue to stir for 12 hours at normal temperature. Usually, in order to prevent the catalyst from being polluted, the mouth of the beaker can be sealed w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| denitrification rate | aaaaa | aaaaa |
| denitrification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com