Dihydropyrimidine derivatives and uses thereof in treatment of HBV infection or of HBV-induced diseases
A compound and selected technology, applied in medical preparations containing active ingredients, antiviral agents, antibody medical components, etc., can solve the problems of constant new infection rate, uncurable treatment, low cure rate and complete suppression of virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
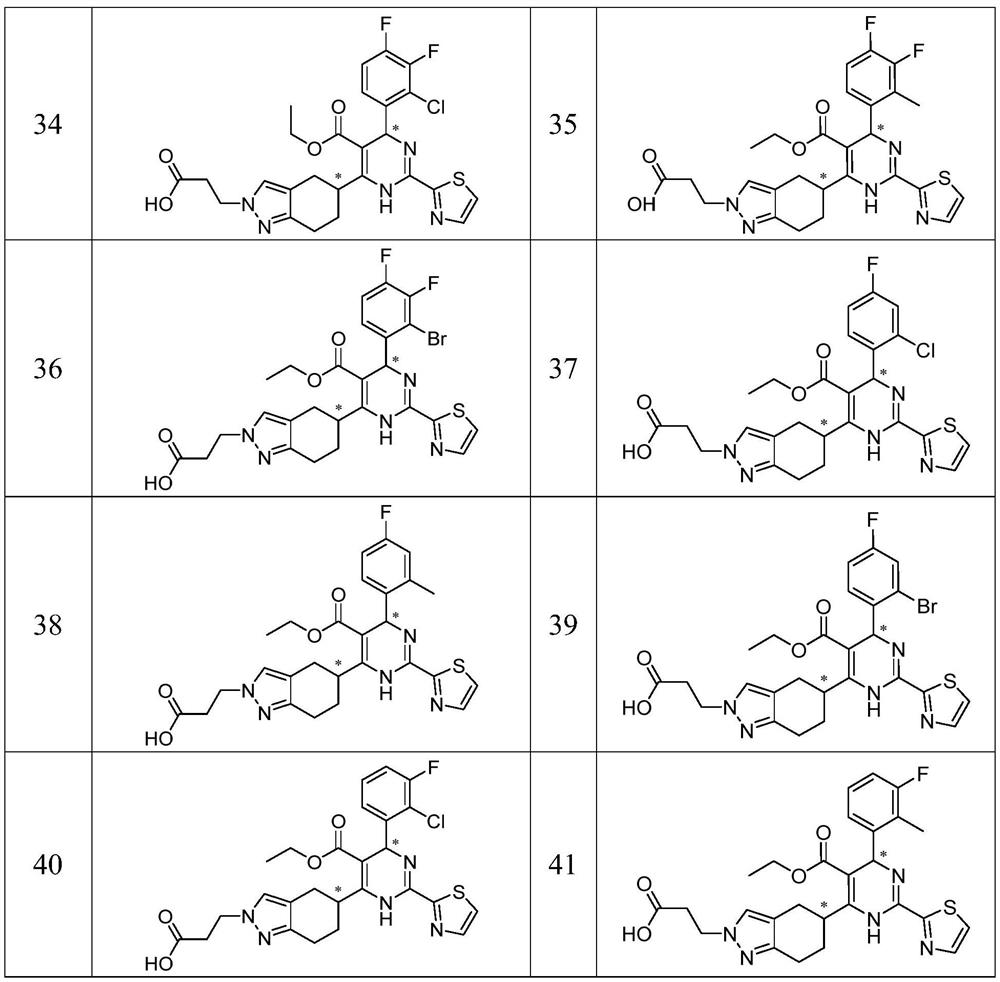
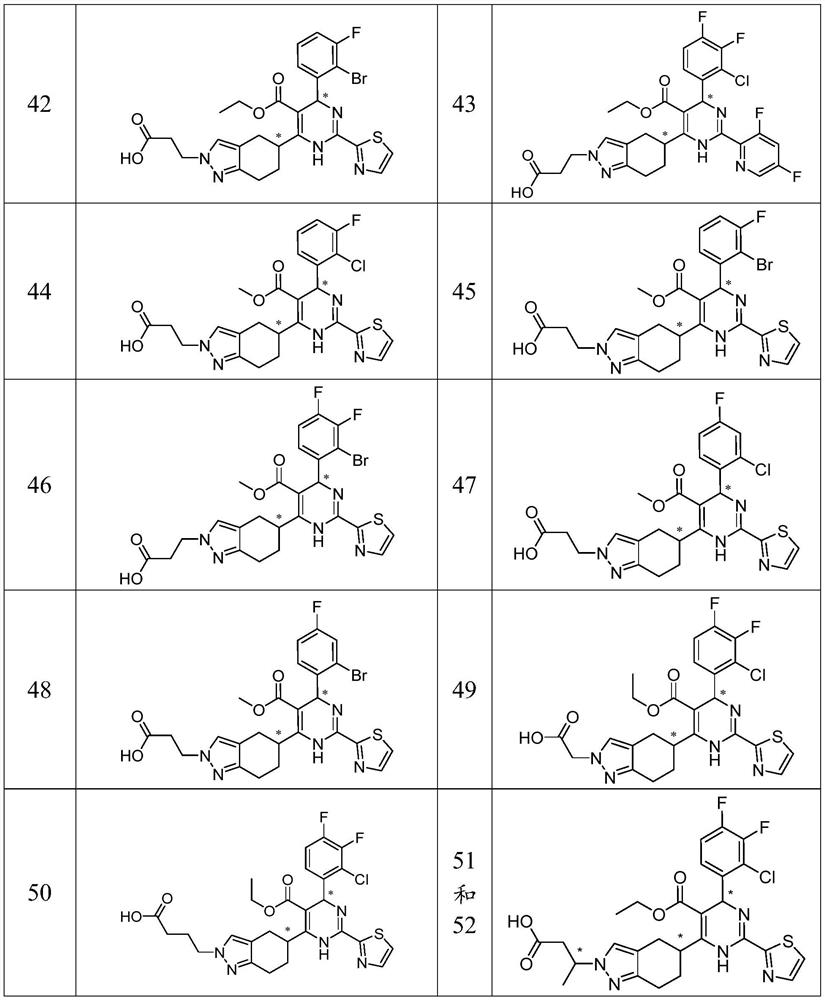
Examples
preparation example Construction
[0114] The preparation of optically active forms is accomplished in any suitable manner, including, by way of non-limiting example, by resolution of racemic forms with recrystallization techniques, synthesis from optically active starting materials, chiral synthesis, or chromatography using chiral stationary phases separation. In one embodiment, a mixture of one or more isomers is used as the disclosed compound described herein. In another embodiment, the compounds described herein contain one or more chiral centers. These compounds are prepared by any means including stereoselective synthesis, enantioselective synthesis or separation of enantiomeric or diastereomeric mixtures. Resolution of the compound and its isomers is accomplished by any means including, by way of non-limiting example, chemical methods, enzymatic methods, fractional crystallization, distillation, and chromatography.
[0115] When the absolute R or S stereochemistry of a compound cannot be determined, it...
example 1
[0198] General solution
[0199]
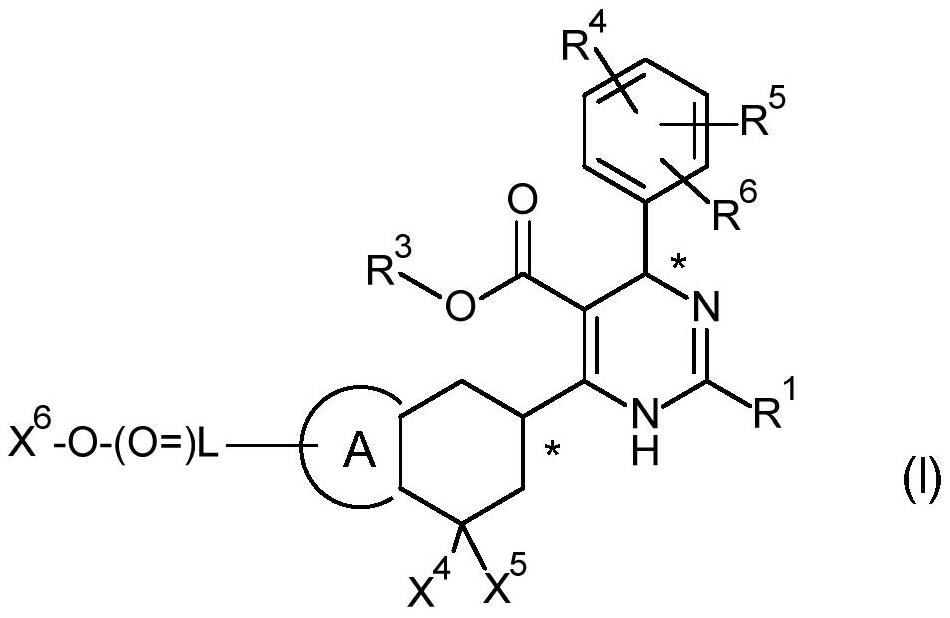
[0200] The general synthesis of compounds of general formula I is described in Scheme 1 and Scheme 2. Compounds of general formula III can be as shown in Scheme 1 (Method A 1 or method A 2 ) described synthesis, the method selection depends on the substituent R on the compound with general formula III 3 . as method A 1 As described in, an acid of general formula II is converted into an active ester by reaction with N,N-carbonyldiimidazole CDI, which is then coupled with ethyl potassium malonate under basic conditions to generate the intermediate, the The intermediates in turn undergo decarboxylation to yield ketoesters of general formula III.
[0201] The final product of general formula I can be synthesized as described in Scheme 2. The former is a chemical method of multi-component reaction with compounds of general formula III, IV and V in a solvent of choice (but not limited to ethanol EtOH) in the presence of a base (but not lim...
example 2
[1293] Example 2: Antiviral assay in HepG2.2.15 cells
[1294] 1. Materials and Equipment
[1295] 1.1. Cell Lines
[1296] HepG2.2.15 (the HepG2.2.15 cell line can be generated by transfection of the HepG2 cell line as described by Sells, Chen and Acs 1987 (Proc. Natl. Acad. Sci. USA [Proceedings of the National Academy of Sciences] 84:1005-1009) described, and the HepG2 cell line can be obtained from In number HB-8065 TM obtained below).
[1297] reagent
[1298] DMEM / F12(INVITROGEN-11330032)
[1299] FBS (GIBCO-10099-141)
[1300] Dimethyl sulfoxide (DMSO) (SIGMA-D2650)
[1301] Penicillin-streptomycin solution (HYCLONE-SV30010)
[1302] NEAA(INVITROGEN-1114050)
[1303] L-Glutamine (INVITROGEN-25030081)
[1304] Geneticin Selective Antibiotic (G418, 500mg / ml) (INVITROGEN-10131027)
[1305] Trypsin Digest (INVITROGEN-25300062)
[1306] CCK8(BIOLOTE-35004)
[1307] QIAamp 96 DNA Blood Kit(12)(QIAGEN-51162)
[1308] FastStart Universal Probe Mast Mix (ROCHE-0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com