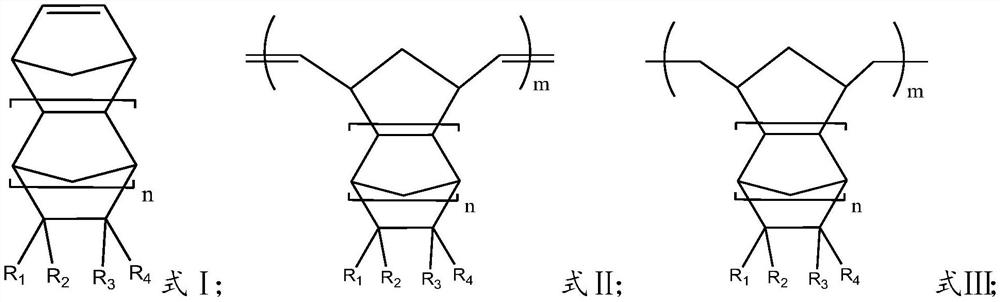
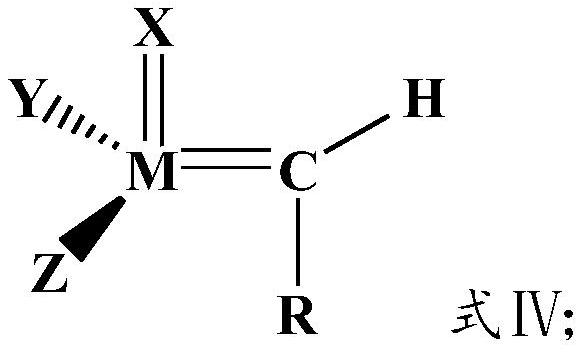
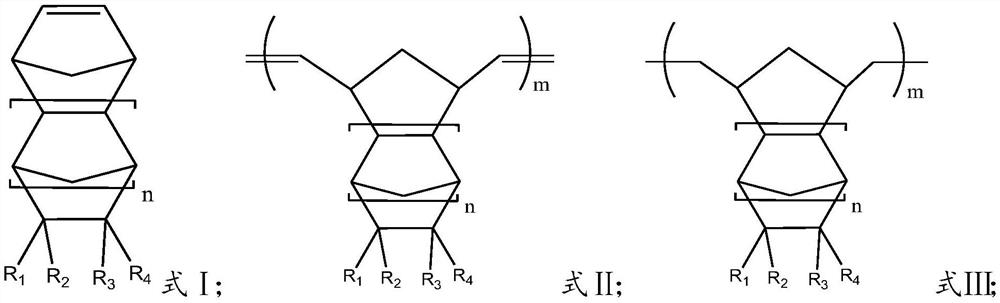
Method for preparing cycloolefin polymer by hydrogenation ring-opening metathesis polymerization method
A cycloolefin polymer and ring-opening metathesis polymerization technology, which is applied in the field of hydrogenation ring-opening metathesis polymerization to prepare cycloolefin polymers, can solve the problems of cumbersome reaction process and achieve good transparency, excellent optical properties, glass The effect of high melting temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 100g of 8-methyl-8-methoxycarbonyltetracyclo[4.4.0.1 2.5 .1 7.10 ]-3-dodecene and 0.268g of 1-hexene were placed in toluene solution, and 60mg of pre-prepared Mo(N-2,6-Pr i 2 C 6 h 3 ) (CHCMe 2 Ph)(O But) 2 Carry out the ring-opening metathesis polymerization reaction at 80°C in an autoclave for 2 hours, then raise the temperature of the reaction system to 165°C, feed hydrogen into the kettle to a pressure of 8MPa and react for 4 hours. Aqueous lactic acid solution was added, and the aqueous phase and catalyst were removed after standing. The resulting solution was added to 3L of isopropanol, and the white polymer was separated by filtration, and dried under vacuum at 50°C for 16 hours to obtain 99 g of polymer. From 1 As can be seen from the H nuclear magnetic detection results, the hydrogen absorption peak at δ=4.5~7.0ppm bonded to the double bond carbon of the main chain disappears, and the hydrogenation ratio is 99.6%; the number average molecular weight Mn i...
Embodiment 2
[0065] 100g of 8-methyl-8-ethoxycarbonyltetracyclo[4.4.0.1 2.5 .1 7.10 ]-3-dodecene and 0.287g of 1-hexene were placed in toluene solution, and 60mg of pre-prepared Mo(N-2,6-Pr i 2 C 6 h 3 ) (CHCMe 2 Ph)(O But) 2 Carry out the ring-opening metathesis polymerization reaction at 80°C in an autoclave for 2 hours, then raise the temperature of the reaction system to 165°C, feed hydrogen into the kettle to a pressure of 8MPa and react for 4 hours. Aqueous lactic acid solution was added, and the aqueous phase and catalyst were removed after standing. The resulting solution was added to 3L of isopropanol, and the white polymer was separated by filtration, and dried at 50°C for 16 hours under vacuum to obtain 98.5 g of polymer. From 1 As can be seen from the H nuclear magnetic detection results, the hydrogen absorption peak at δ=4.5~7.0ppm bonded to the double bonded carbon of the main chain disappears, and the hydrogenation ratio is 99.7%; the number average molecular weight M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com