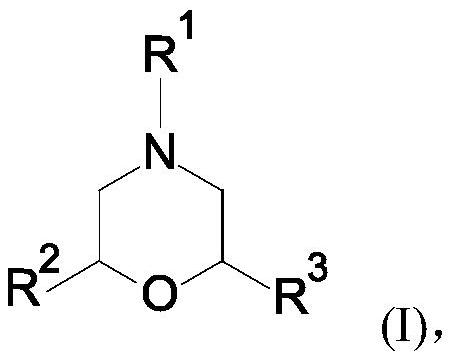
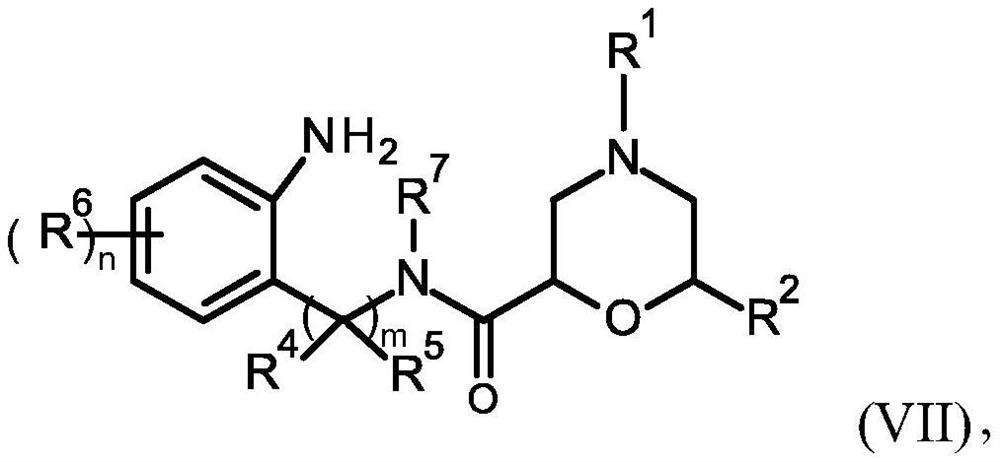
Novel cyclic amidine compounds for treatment of autoimmune disease
A compound, selected technology, applied in allergic diseases, organic chemistry, drug combination, etc., can solve the problem of no steroid-free and non-cytotoxic oral drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0108] The present invention will be more fully understood by reference to the following examples. However, they should not be construed as limiting the scope of the invention.
[0109] abbreviation
[0110] The present invention will be more fully understood by reference to the following examples. However, they should not be construed as limiting the scope of the invention.
[0111] The abbreviations used in this article are as follows:
[0112] ACN: Acetonitrile
[0113] Boc 2 O: di-tert-butyl dicarbonate
[0114] Tf 2 O: trifluoroformic anhydride
[0115] DCM: dichloromethane
[0116] DDI drug-drug interactions
[0117] DIPEA diisopropylamine
[0118] DMA Dimethylacetamide
[0119] EA or EtOAc: ethyl acetate
[0120] FA: formic acid
[0121] HATU 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
[0122] HLM human liver microsomes
[0123] hr hours
[0124] hrs hours
[0125] IC 50 : half inhibitory concentratio...
preparation example
[0151] The following examples are intended to illustrate the implication of the present invention, but in no way represent the limitation of the implication of the present invention:
example 1
[0153] 5-[(2R,6R)-2-(5-bromo-3,4-dihydroquinazolin-2-yl)-6-methyl-morpholin-4-yl]quinoline-8-carbonitrile
[0154]
[0155] The title compound was prepared according to the following scheme:
[0156]
[0157] Step 1: Preparation of (2R,6R)-6-methylmorpholine-2-carboxylic acid methyl ester hydrochloride (compound 1b)
[0158] At rt, to (2R,6R)-4-tert-butoxycarbonyl-6-methyl-morpholine-2-carboxylic acid (Compound 1a, CAS: 1581752-93-3, WuXi AppTec (Tianjin) New Drug Development Ltd., catalog number: RC-160325, 1.5 g, 6.1 mmol) in MeOH (20 mL) was added dropwise with SOCl 2 (1 mL). The reaction mixture was heated to reflux for 2 hrs, then cooled to rt, and concentrated under vacuum to give crude compound 1b (1.2 g, 100% yield) as a white solid. 1 HNMR (400MHz, DMSO-d 6 ) δ = 9.63 (br s, 2H), 4.55 (br d, J = 10.88Hz, 1H), 3.95 (br dd, J = 10.21, 5.93Hz, 1H), 3.41 (br d, J = 10.64Hz, 1H ), 3.22(br d, J=11.98Hz, 1H), 2.94(t, J=12.10Hz, 1H), 2.65(t, J=11.92Hz, 1H), 1.16(d,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com