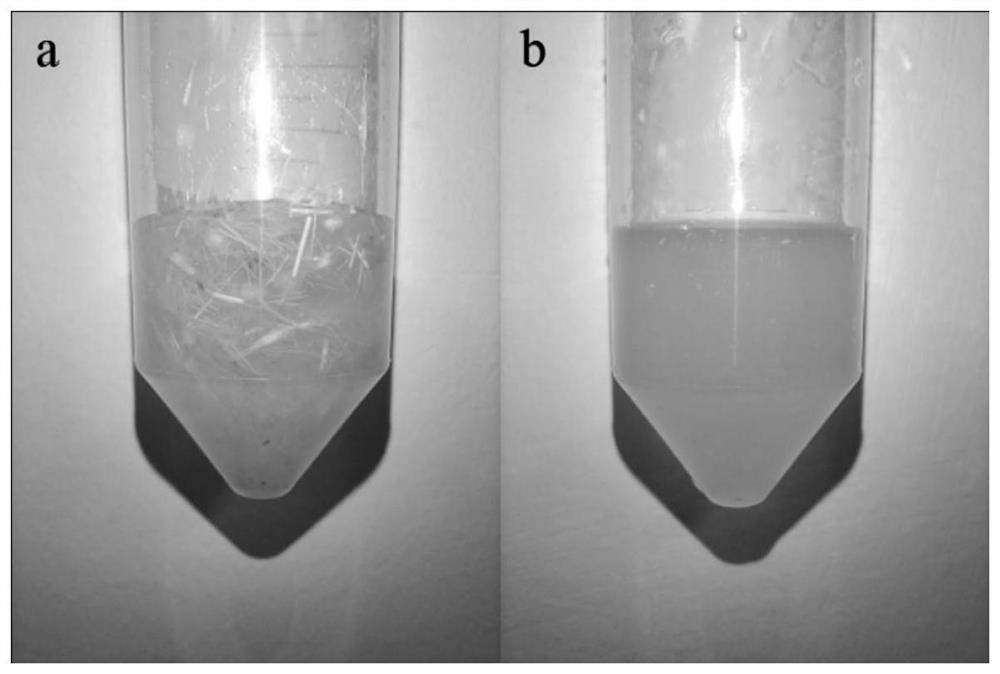
Keratinase mutant with improved low-temperature enzymolysis performance, and application thereof
A technology of keratinase mutation and keratinase, which is applied in the field of keratinase mutants to achieve the effects of reducing costs, reducing heating process requirements, and avoiding loss of vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Construction of keratinase error-prone PCR mutants
[0049] Specific steps are as follows:
[0050] 1. Construction of recombinant plasmid pP43NMK-kerB
[0051] (1) Chemically synthesized nucleotide sequence as shown in SEQ ID NO.1 can be used to produce the gene of keratinase (by the gene of coding signal peptide, the gene of coding leader peptide, 6×His tag and the gene of coding keratinase in sequence obtained in series); and the obtained gene was ligated with the pP43NMK plasmid using a homologous recombination kit (Clonexpress II One Step Cloning Kit) to obtain a ligation product.
[0052] (2) Transform the ligation product into Escherichia coli JM109, spread the transformed product on LB solid medium, culture at 37°C for 12-14 hours, pick 4 transformants on the LB solid medium, insert them into LB liquid medium for culture, After culturing at 37°C for 12 hours, the plasmid was extracted, and the extracted plasmid was verified by enzyme digestion and s...
Embodiment 2
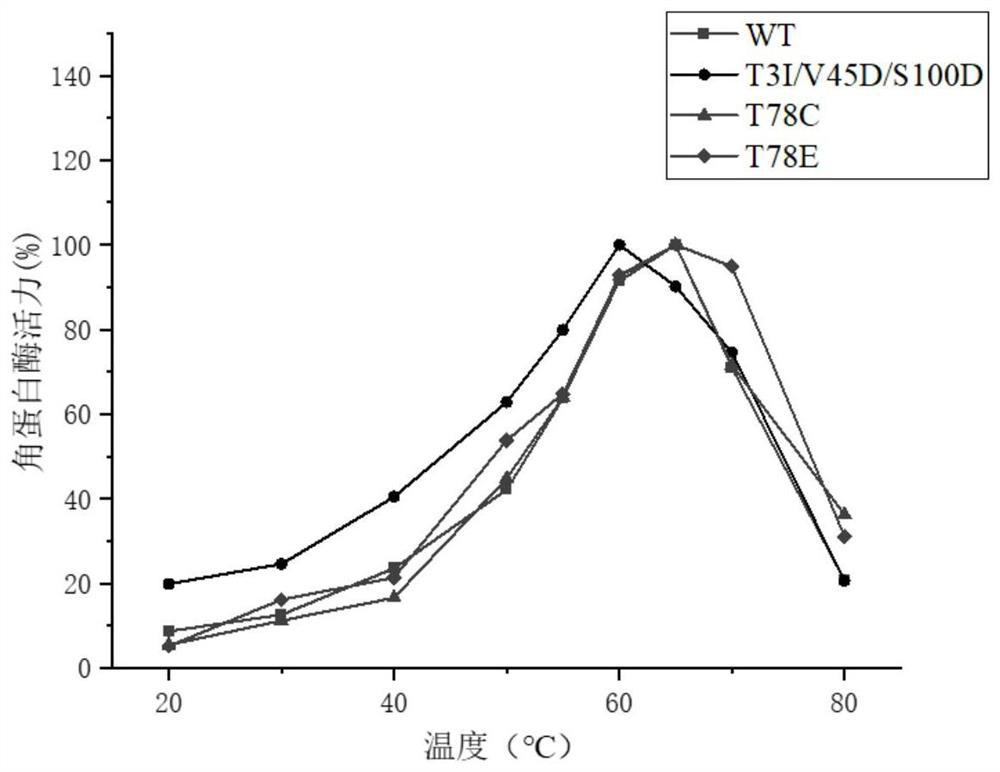
[0070] Embodiment 2: Construction of recombinant bacteria and screening of mutants
[0071] Specific steps are as follows:
[0072] (1) The recombinant plasmid containing the mutant gene obtained in Example 1 and the recombinant plasmid pP43NMK-kerB containing the wild-type enzyme were respectively transformed into Bacillus subtilis (Bacillus subtilis) WB600 to obtain transformation products respectively, and respectively coated the transformation products on the additive Kanamycin (final concentration 50mg L -1 ) LB solid medium, cultivated at 37°C for 8h, respectively inoculated a large number of single colonies obtained into 96 deep-well plates containing fermentation medium, cultivated at 37°C and 220rpm for 24h, and obtained the fermentation broth containing mutants and Fermentation broth containing wild-type keratinase;
[0073] (2) Centrifuge the fermentation broth obtained in step (1) at 4° C. and 4000 rpm for 20 min to obtain fermentation supernatants respectively. ...
Embodiment 3
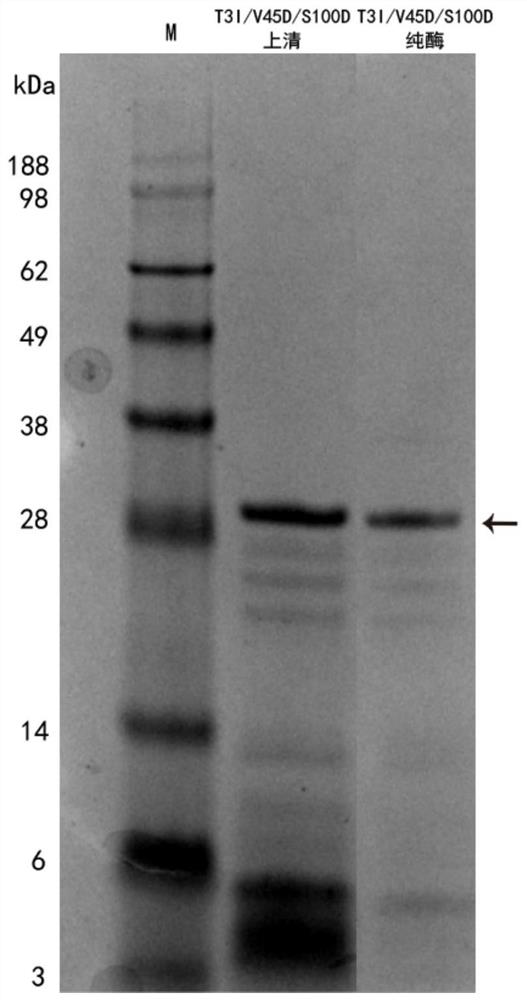
[0080] Embodiment 3: Separation and purification of keratinase mutant
[0081] Specific steps are as follows:
[0082] Purification of keratinase: AKTAavant protein purifier was used for purification of recombinant protein. Since the keratinase mutants are all added with a histidine tag, they can be separated and purified using a nickel ion affinity chromatography purification column, and the specific steps are as follows:
[0083] (1) Equilibration: equilibrate the purification column with 5 times the volume of 20mmol / L pH 7.4 Tris-HCl buffer;
[0084] (2) Sample loading: the pre-treated sample is loaded at a flow rate of 0.5ml / min, and the sample loading volume is generally not more than 5 times the column volume;
[0085] (3) Elution: including elution of unadsorbed substances, miscellaneous proteins and target proteins, the flow rate is 2.0mL / min, the eluent is 20mmol / L Tris-HCl buffer solution with pH 7.2 containing 50mM imidazole, and the elution is carried out with 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com