A kind of preparation method of posaconazole intermediate
A technology for posaconazole and intermediates, applied in the field of medicinal chemistry, can solve the problems of harsh synthesis conditions, severe reaction conditions, and many reaction steps, and achieve the effects of low cost, high reaction efficiency, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The technical solutions in the embodiments of the present invention will be clearly and completely described below. Obviously, the described embodiments are only some embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative efforts shall fall within the protection scope of the present invention.
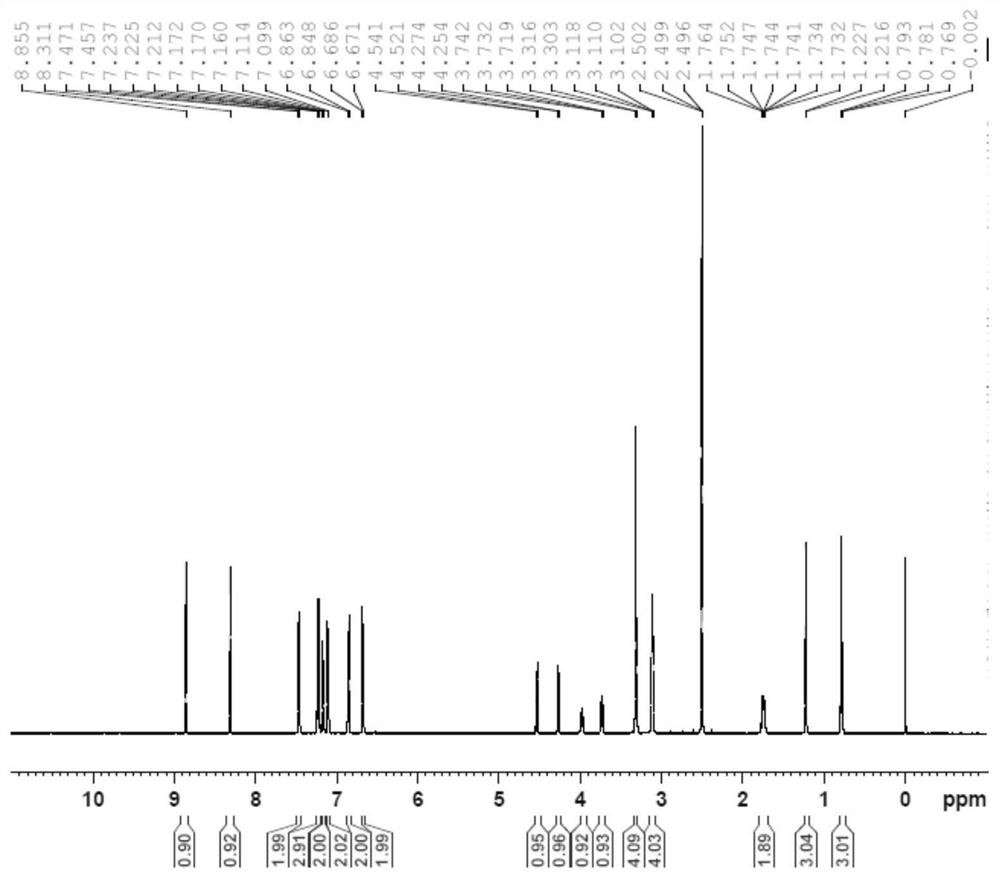
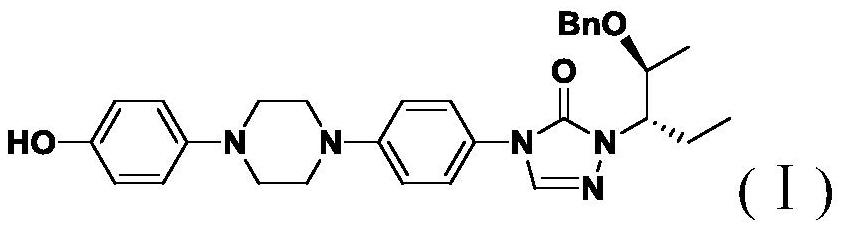
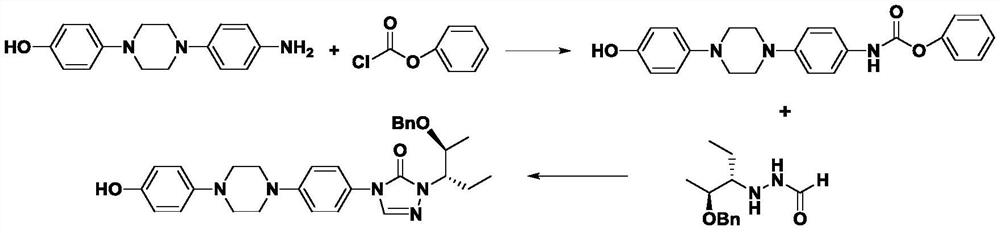
[0029] Example Synthesis of Posaconazole Intermediates Represented by Formula I
[0030] The synthetic route is shown below:
[0031]
[0032] S1: Add 5000 mL of dimethylacetamide, 100 g of anhydrous piperazine, 516 g of p-bromonitrobenzene, and 353 g of potassium carbonate powder in sequence to a dry reaction flask under nitrogen protection, and heat to reflux at 100-200 °C under vigorous stirring after the addition. 16h, cooled, filtered, the filtrate was concentrated under reduced pressure, and the residue was recrystallized from a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com