Application of CDN1163 in preparation of medicine for relieving or treating neuropathic pain
A CDN1163, neurological technology, applied in the field of neurological drugs, can solve problems such as inability to infer, and achieve the effects of inhibiting the increase in neuronal excitability, improving activation, and improving mechanical pain sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]This example is intended to illustrate the use of CDN1163 by CDN1163 to reduce chronic sciatic injury, CCI) rat mechanical pain threshold and heat pain in the preparation of mitigation or therapeutic neuropathic pain drug.
[0036]Experimental animal
[0037]Purchased from 50 male SD rats in Qinglong Mountain, Nanjing, Jiangning District, where the rats were placed in a quiet and removal of strong light, and the light and dark time ratio were 1: 1, Room temperature was controlled at 22-25 ° C, humidity, and rats were free to eat and drink water.
[0038]2. Experimental drugs and consumables
[0039]
[0040]3. Experimental equipment and equipment
[0041]
[0042]Glass separation, sterilization gauze, tissue, cotton swab, trace syringe.
[0043]4. Experimental method
[0044]4.1 Random Group
[0045]Take 10 cages with marker pen, the animal temporary number is the first cage 1 ~ 5, the second cage 6 ~ 10, sequentially to the 10th cage 46 ~ 50, randomly crawled the rat weighing, each cage Placed 5, and use ...
Embodiment 2
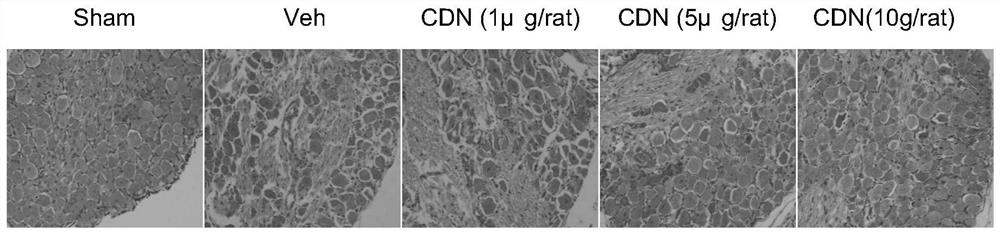
[0056]This example is used to illustrate the improved L of the CDN1163 provided by the present invention.5 Application of DRG form in the preparation of mitigation or treatment of neuropathic pain drugs.
[0057]Experimental drugs and consumables
[0058]
[0059]2. Experimental equipment and equipment
[0060]
[0061]3. Experimental method
[0062]3.1 Application materials and fixation
[0063]Dead rats at the broken head intercept L4-6Section of the spine, choose L5 DRG is rinsed in a pre-cooled saline, and it is immediately invested in 4% polyfethaldehyde to secure overnight.
[0064]3.2 Dehydration and transparency
[0065]The tissue was placed in 75%, 85%, 95%, 100%, 100% of the dehydration. The dehydrated tissue was placed in two pure xylenes to be transparent.
[0066]3.3 Diffility and embedding
[0067]The tissue placed in xylene was placed in an oven at a 40 ° C for 40 min, then placed in two new paraffin, 0.5 h each time. Pour the dissolved paraffin into the first prepared container, quickly clip the tis...
Embodiment 3
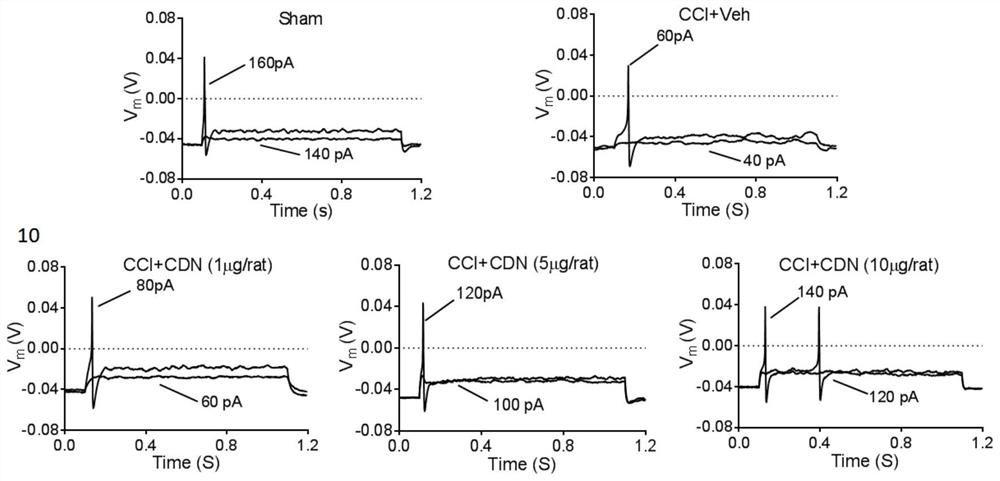
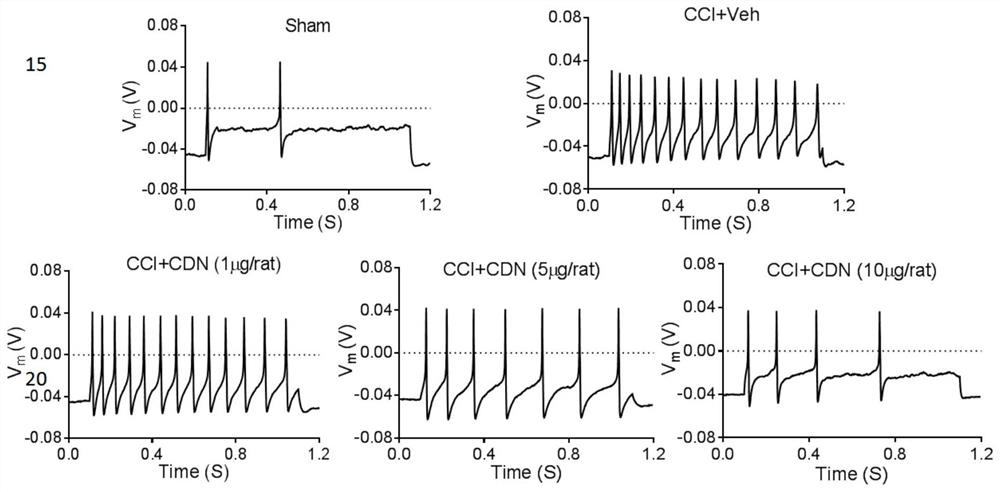
[0081]This example is used to illustrate the CDN1163 inhibition of the present invention.4-6 The use of DRG neuronal excitability in the preparation of mitigation or treatment of neuropathic pain drugs.
[0082]Experimental drugs and consumables
[0083]
[0084]
[0085]2. Experimental equipment and equipment
[0086]
[0087]3. Experimental solution formulation (reagents were purchased from Sigma, USA)
[0088]3.1 Action potential AP cell fluid
[0089]The formulation is 140 mm KCl, 0.5 mM EGTA, 3mm Mg-ATP, 5MM HEPES, using KOH to adjust pH to 7.4. That is, 521.85 mg KCl, 9.51 mg EGTA, 76.08 mg mg-ATP, 59.58 mg HEPES were added to 40 ml, using KOH to adjust pH to 7.3-7.4, to 50 mL, 0.22 μm filter filtration, 4 ° C Preservation .
[0090]3.2 Action potential AP extracellular fluid
[0091]Formula 140mm NaCl, 3mm KCl, 2mm CaCl22mm mgcl2, 10mm HEPES, PH 7.4. That is, 4090.8 mg NaCl, 111.83mg KCl, 110.98mg CaCl295.21mg MGCL21191.55 mg HEPES was added to 400 mL, and NaOH was used to adjust pH to 7.3-7.4, and the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com