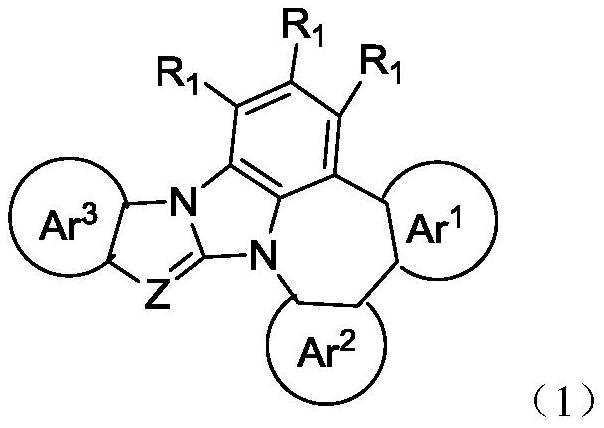
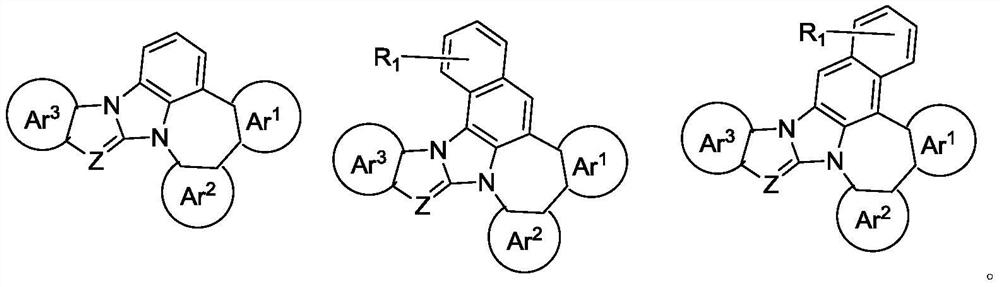
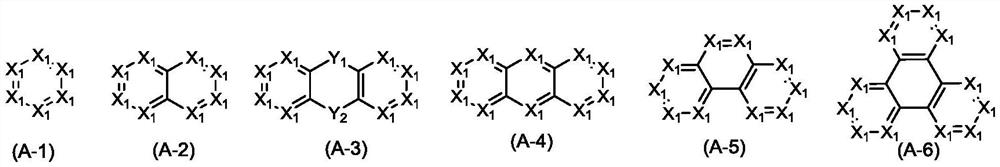
Nitrogen-containing heterocyclic organic compound and application thereof
A technology of organic compounds and mixtures, applied in the field of electroluminescent materials, can solve the problems of device performance and lifespan that need to be improved, and achieve the effects of improving luminous efficiency and lifespan, improving electroluminescent efficiency and device lifespan, and reducing manufacturing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195]
[0196] Synthesis of Intermediate 1-3: Dissolve 1-1 (20.0g) and 1-2 (43.8g) in dry DMF, add cesium carbonate (38.46g), and react at 140°C for 8h. After cooling, most of the solvent was distilled off under reduced pressure, the residual reaction liquid was washed with water and extracted, and the organic phase was rotary evaporated to remove the solvent and then recrystallized to obtain intermediate 1-3.
[0197] Synthesis of Compound 1: Intermediate 1-3 (10.0g), Pd(PCy 3 ) 2 Cl 2 (CAS 78655-99-9, 1.2g), isovaleric acid (1.1g), and cesium carbonate (10.0g) were added to 100ml of dry DMAC, and stirred at 170°C for 6h under a nitrogen atmosphere. After cooling, most of the solvent was distilled off under reduced pressure, extracted and washed with water to separate the liquids, and the organic phase was rotary evaporated to remove the solvent and then recrystallized to obtain compound 1. MS (ASAP):602.
Embodiment 2
[0199]
[0200] Synthesis of Intermediate 2-3: Intermediate 2-1 (20.0g), 2-2 (16.6g), DMAP (3.17g) were dissolved in dry DMSO, and cesium carbonate (33.0g) was added, under nitrogen Under the atmosphere, it was heated to 100°C for 8h. After cooling, most of the solvent was distilled off under reduced pressure. The residual reactant was washed with water and extracted, and the organic phase was rotary evaporated to remove the solvent and then recrystallized to obtain intermediate 2-3.
[0201] The synthesis of material 2 refers to the synthesis of material 1 in Example 1, except that 1-3 is replaced by 2-3. MS (ASAP): 588.
Embodiment 3
[0203]
[0204] Synthesis of intermediate 3-2: 3-1 (15.0g), biboronic acid pinacol ester (9.3g), Pd(dppf)Cl 2 ([1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, 1.2g), potassium acetate (4.9g) were added to 1,4-dioxane, under nitrogen atmosphere, 100 ℃ reaction 8h. After cooling, most of the solvent was distilled off under reduced pressure, and the remaining reaction liquid was washed and extracted with water, and the organic phase was rotary evaporated to remove the solvent, and then recrystallized to obtain intermediate 3-2.
[0205] Synthesis of intermediate 3-4: 3-2 (10.0g), 3-3 (5.2g), Pd(PPh 3 ) 4 (Tetrakis(triphenylphosphine)palladium, 0.8g) and potassium carbonate (4.2g) were added to 1,4-dioxane, and an appropriate amount of water was added. The reaction was carried out at 100°C for 8h under nitrogen atmosphere. After cooling, most of the solvent was distilled off under reduced pressure, and the remaining reaction solution was washed and extracted wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com