Process for producing trifluoroiodomethane
A technology for trifluoroiodomethane and trifluoroacetyl, which is applied in the gas phase field for producing trifluoroiodomethane, and can solve problems such as reduced process efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0054] Preparation of trifluoroiodomethane according to Equation 1
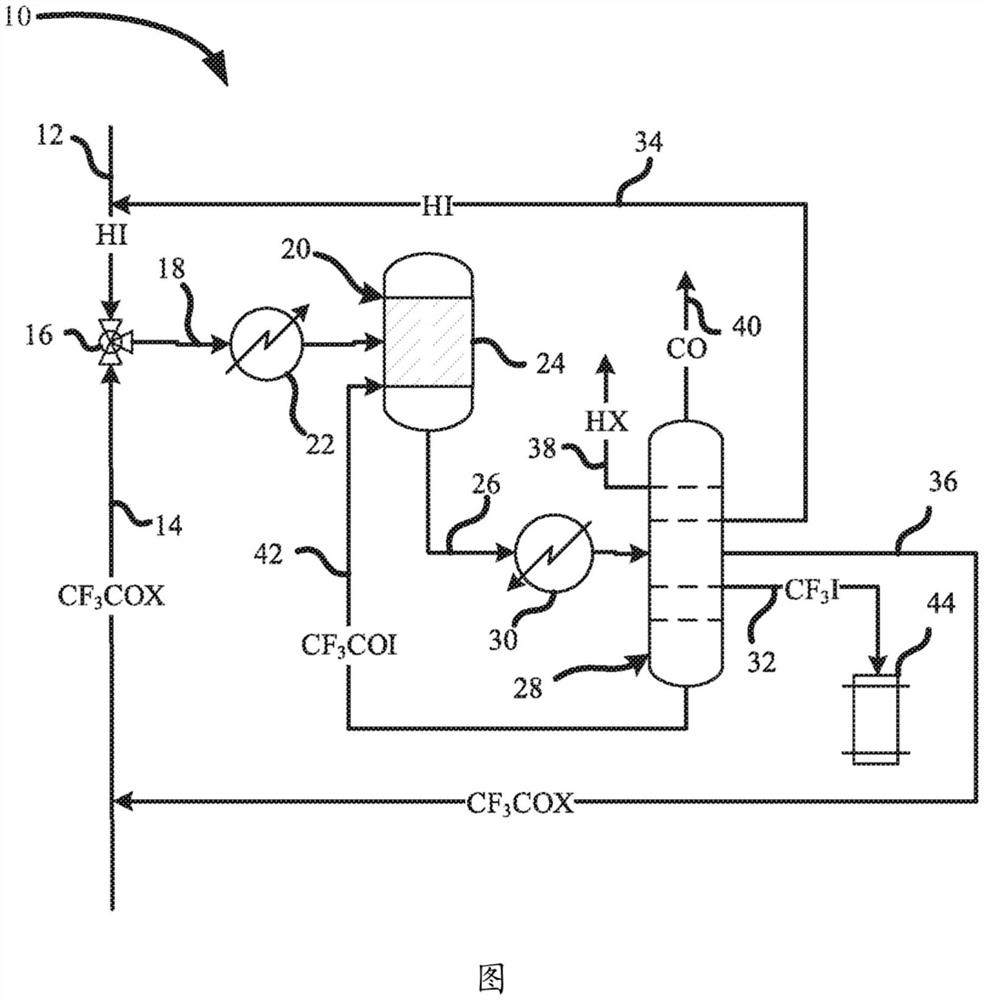
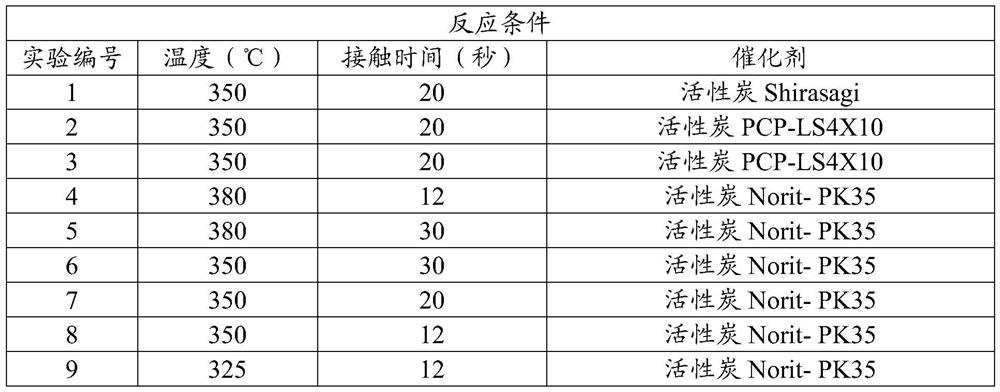
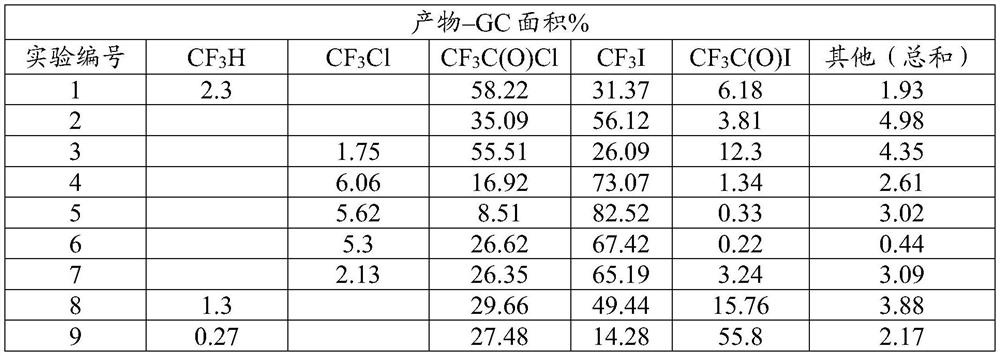
[0055] In this example, the production of iodotrifluoromethane from hydrogen iodide and trifluoroacetyl chloride according to Equation 1 as described above is demonstrated. In nine series of experiments, equimolar amounts of trifluoroacetyl chloride and anhydrous hydrogen iodide were passed through a preheater and heated to a temperature of about 100°C. The heated reactants were then passed through a stainless steel tube 3 / 8 inch (9.5 mm) in diameter and 6 inches (152 mm) in length. Depending on the experiment, the tubes were heated to a temperature ranging from 350°C to 380°C, and were purged with nitrogen for at least one hour before each experiment to remove any water. For each experiment, the tubes contained one of several catalysts. Contact times range from 12 seconds to 30 seconds. All vented vapors from each experiment were collected in sample bags for GC and GC-MS analysis. The results are shown...
Embodiment 2
[0064] Embodiment 2: the separation of trifluoroiodomethane
[0065] In this example, the isolation of trifluoroiodomethane is demonstrated. A mixture comprising 85% by weight iodotrifluoromethane, 10% by weight trifluoroacetyl iodide and 5% by weight carbon monoxide may be fed to the distillation column. The distillation column may include a 10 gallon reboiler, 2 inch ID 10 ft. column and about 30 theoretical plates. Distillation columns can be equipped with temperature, absolute and differential pressure transmitters. The distillation may be run at a pressure of about 275 KPa and the condenser at a temperature of about -13°C to collect the trifluoroiodomethane.
[0066] aspect
[0067] Aspect 1 is for producing trifluoroiodomethane (CF 3 I) The gas phase process comprising providing a reactant stream comprising hydrogen iodide and a trifluoroacetyl halide selected from the group consisting of trifluoroacetyl chloride, trifluoroacetyl fluoride, trifluoroacetyl bromi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com