Application of diagnostic marker for newborn biliary atresia
A diagnostic marker and biliary atresia technology, applied in the field of biomedicine, can solve the problems of limited accuracy, large differences in children, and many factors affecting platelet measurement, and achieve high accuracy, reliable detection results, and low detection limit. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1 The establishment and optimization of liquid chromatography-tandem mass spectrometry method
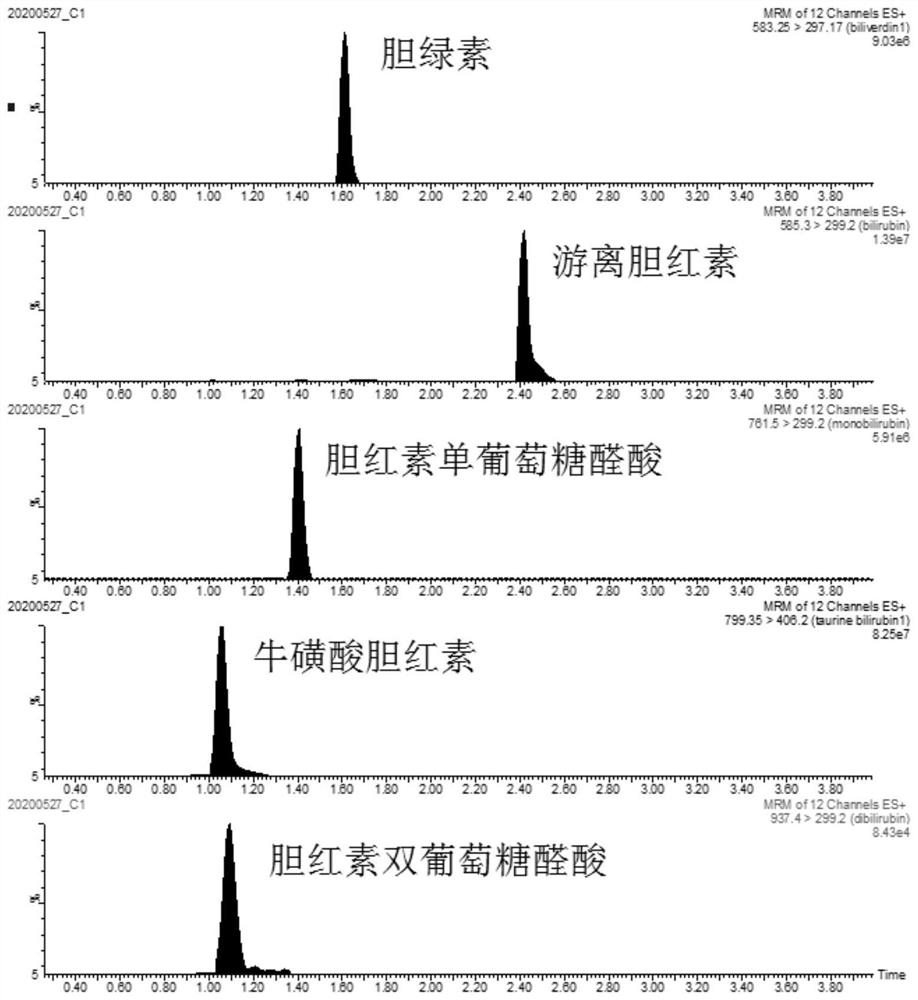
[0088] In this example, the establishment process of the qualitative and quantitative detection method for the components of bilirubin in biological samples based on liquid chromatography-tandem mass spectrometry is described for the purpose of illustration, including selection of liquid phase conditions, setting of mass spectrometry conditions, preparation of standard solutions , Optimizing the analysis method, drawing a standard curve. The detection method of this embodiment has high sensitivity and high selectivity, strong anti-interference ability, and can become an effective tool for qualitative and quantitative analysis of bilirubin components.
[0089] Bilirubin samples contain a variety of components. The inventor found that when the pH value of the mobile phase A of liquid chromatography was 3-4.5, the main components in the bilirubin sample were free bil...
Embodiment 2
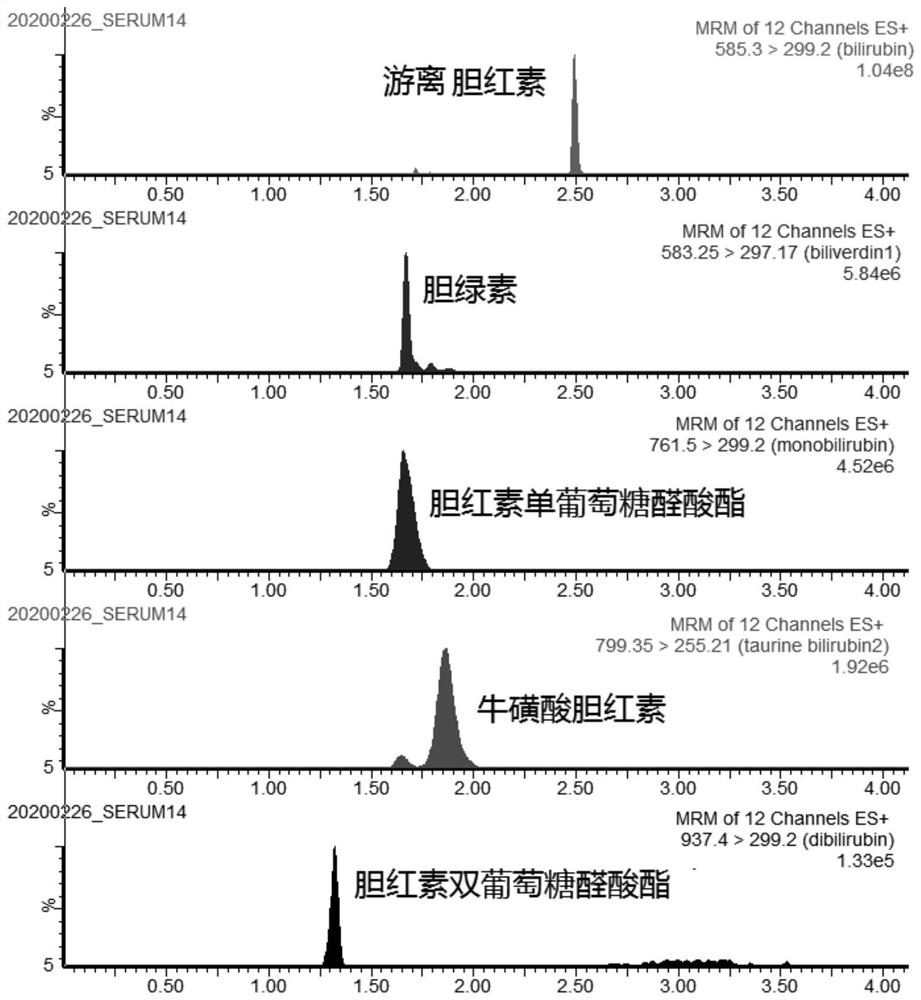
[0112] Qualitative and quantitative determination of bilirubin in embodiment 2 blood samples
[0113] Sampling: Take the serum, plasma and whole blood samples of 3 test subjects, and each sample is divided into 3 parts, such as serum 01, serum 02, serum 03, etc., a total of 9 biological samples.
[0114] Biological sample processing:
[0115] Serum 01: Take 20 microliters of samples, add 10 microliters of bilirubin taurine as an internal standard, and then add 80 microliters of methanol:acetonitrile (volume ratio 1:1) solution containing 1mg / ml BHT and 200mmol / L ascorbic acid , Shake at 1450 rpm for 20 minutes at 10°C in the dark. Centrifuge at 18,000 g for 20 minutes at 4°C. Take 60 microliters of supernatant to a 96-well plate, and use it as a detection sample for high performance liquid chromatography tandem mass spectrometry detection.
[0116] Serum 02: Take 20 microliters of samples, add 10 microliters of bilirubin taurine as an internal standard, then add 80 microlit...
Embodiment 3
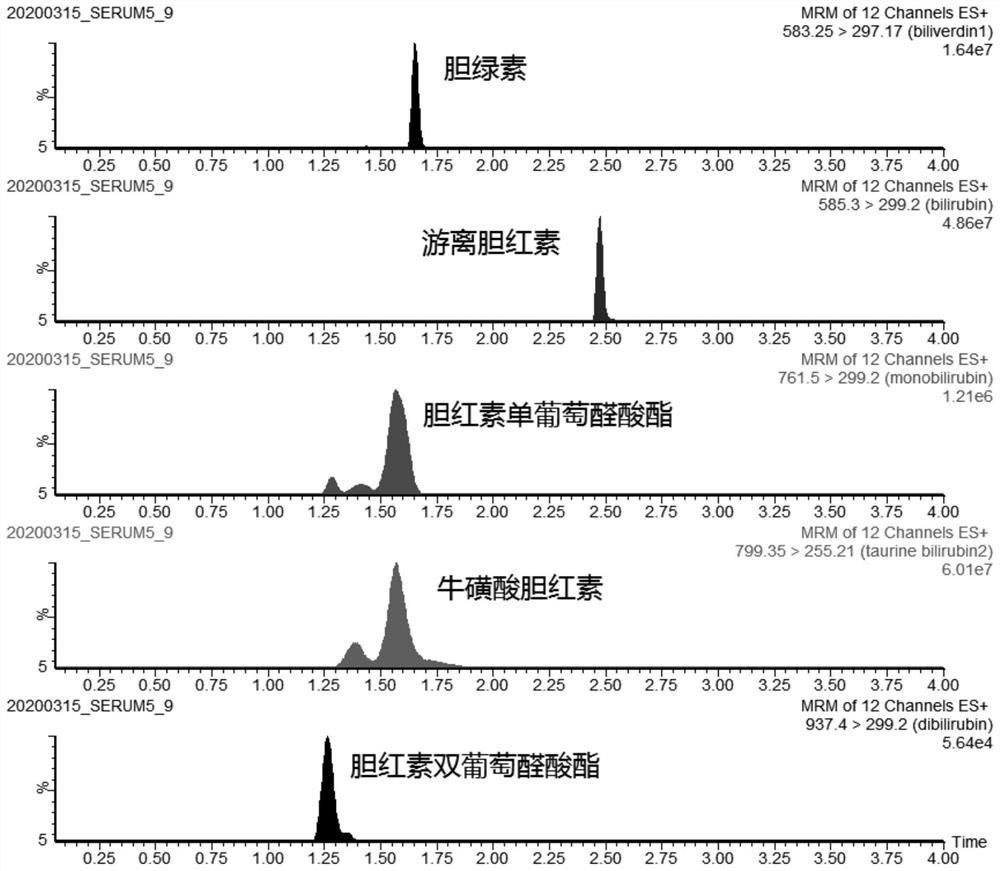
[0128] Qualitative and quantitative determination of bilirubin components in embodiment 3 dried blood spots
[0129] Dried blood spot sample preparation
[0130] Fingertip blood was collected from newborns within 4 days of birth to prepare dry blood spot samples. Proceed as follows:
[0131] a) Pretreatment of filter paper: Soak 903 filter paper in ethanol solution containing 1mg / mL BHT and 200mmol / L ascorbic acid repeatedly for 2 minutes, take it out and drain, then dry the filter paper naturally in the shade for later use.
[0132] b) Preparation: Take a suitable test tube and add an appropriate amount of diluent. Take a micropipette and connect it with a latex tip, check whether there is any air leakage at the connection, or take a disposable micropipette (siphon principle), blood collection needle, 75% ethanol or iodophor, cotton swab, etc. for backup.
[0133] c) Massage: Gently massage the central part of the ulnar side of the ring finger of the left hand with more mu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com