Preparation method of PF-06651600 intermediate
A reaction time and compound technology, applied in the preparation of organic compounds, sulfonate ester preparation, organic chemical methods, etc., can solve problems such as hidden dangers, high price, and violent heat release of resolving agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
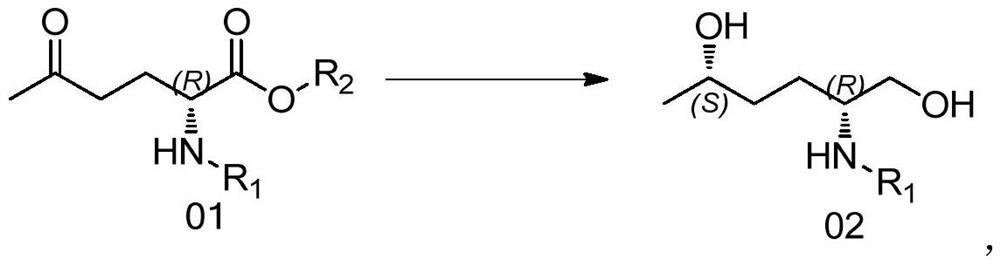
[0062] Example 1 Preparation of (R)-2-(N-Boc-amino)-5-carbonyl-hexanoic acid ethyl ester (compound 01-1)
[0063]
[0064] Under the protection of nitrogen, add Boc-D-ethyl pyroglutamate (compound 00-1) 60g (1.0eq, 233.2mmol) and THF 500ml to a 1000ml three-necked flask successively, stir and dissolve at room temperature and then cool down to - At 40°C, slowly add 110ml (1.4eq, 330mmol) of the MeMgBr format reagent dropwise. After the drop, slowly raise the temperature to -20°C, and monitor the reaction by TLC. After the raw materials have reacted, add dropwise 300ml of saturated NH 4 Quench the Cl solution, separate the liquids, extract the aqueous phase with 300ml of ethyl acetate, wash the combined organic phase with 500ml of water once, spin dry under reduced pressure, add 300ml of n-heptane to cool down and crystallize, stir, filter, and dry to obtain (2R)- 63.7 g of off-white solid 2-(N-Boc-amino)-5-carbonyl-hexanoic acid ethyl ester (compound 01-1), yield 93%. analy...
Embodiment 2
[0067] Example 2 Preparation of (2S,5R)-2-(N-Boc-amino)-1,5-hexanediol (compound 02-1)
[0068]
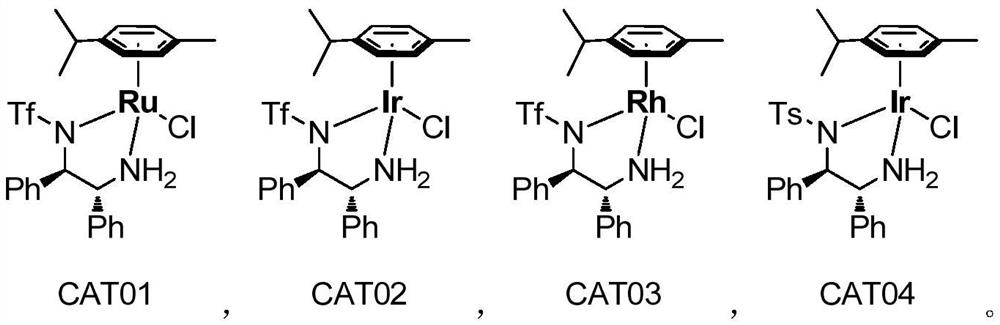
[0069] At room temperature, 10.92g (1.0eq, 40mmol) of compound 01-1 and 40ml of DMF were added to a 100ml two-necked flask, stirred and dissolved, and 13.7ml (6eq, 120mmol) of glacial acetic acid, 13.3ml (2.4eq, 96mmol) of glacial acetic acid were added under nitrogen protection. ) TEA and 0.124g (0.005eq, 0.2mmol) Ru chiral catalyst (CAT01), reacted at 20°C-50°C for 24h, TLC monitored the reaction, the raw materials were completely reacted, after the reaction, neutralized with saturated NaHCO3 solution, Add 80ml of EA for extraction three times, wash once with 160ml of water, dry the organic phase with anhydrous sodium sulfate, spin dry the organic phase under reduced pressure to obtain a colorless oil, add n-heptane for low-temperature crystallization, suction filter, and vacuum-dry the solid to obtain 7.46 g of white solid (2S,5R)-2-(N-Boc-amino)-1,5-hexanediol (compound 02-...
Embodiment 3
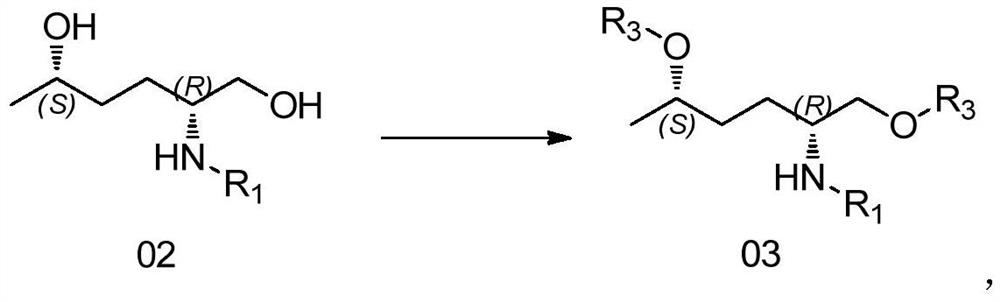
[0073] Example 3 Preparation of (2R,5S)-1,5-dimethylsulfonate-2-(N-Boc-amino)hexane (compound 03-1)
[0074]
[0075] Under nitrogen protection, 6g (1.0eq, 25.7mmol) of compound 02-1 and 36ml of DCM were added to a 100ml two-necked flask, stirred and dissolved at room temperature, cooled to 0°C, 6.5g (2.5eq, 64.3mmol) of TEA was added, stirred 0.5h, control until the reaction temperature is less than 0°C, add 6.5g (2.2eq, 56.7mmol) MsCl solution in DCM dropwise, keep warm for reaction after dropping, TLC monitors the reaction, the raw materials react completely (about 1-2h), add 40ml of water Quench the reaction, separate the layers, wash the DCM layer once with 40ml saturated NaHCO3 and aqueous solution, and rotary evaporate to dryness under reduced pressure at room temperature to obtain 9.8g of (2R,5S)-1,5-dimethylsulfonic acid as a yellow oil -2-(N-Boc-amino)hexane (compound 03-1), yield 98%. analyze data:
[0076] LC-MS: m / z (ESI): 412.0 (M+Na + ) + ;
[0077] 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com