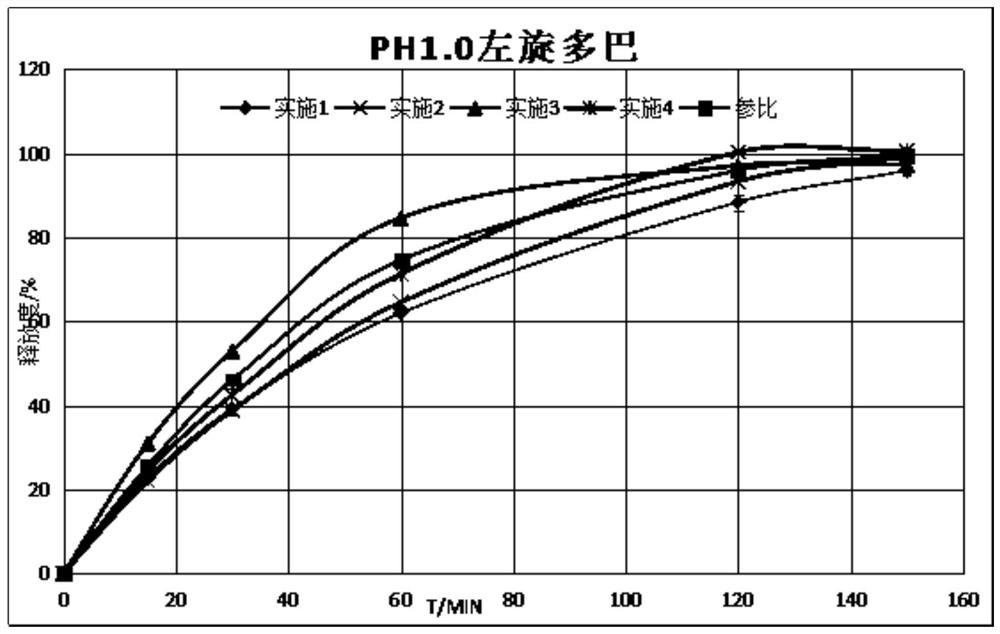
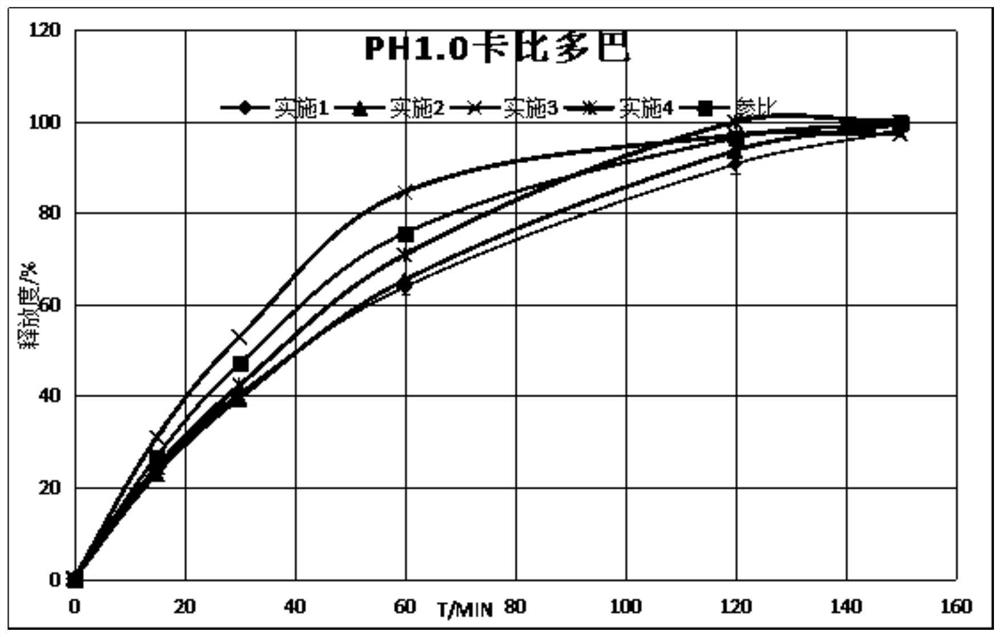
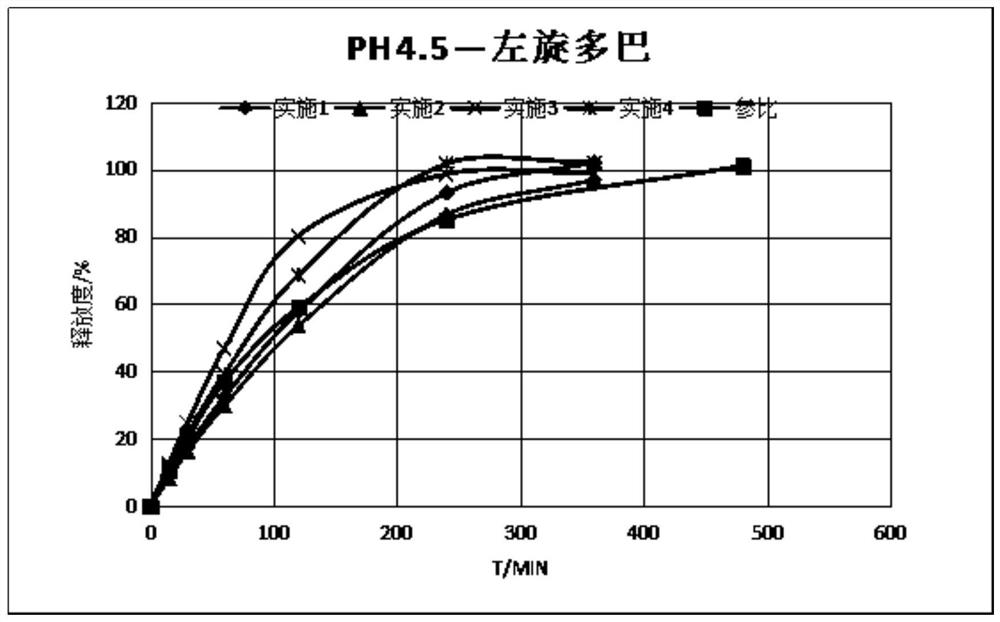
Carbidopa-levodopa sustained-release tablet and preparation method thereof
A technology for sustained-release tablets and levodopa, which is applied in the field of medicine, can solve the problems of difficult production process, difficult industrialized production and the like, and achieves the effects of good color, easy industrialized production, and alleviation of adverse reactions in the surrounding area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of carlevodopa slow-release tablet, comprises the following steps,
[0052] (1) mixing,
[0053] The carbidopa, levodopa and slow-release material that take prescription quantity are mixed homogeneously;
[0054] (2) Adhesive preparation,
[0055] Weigh the prescribed amount of adhesive, add an appropriate amount of water and / or ethanol, stir to form a solution, and set aside;
[0056] (3) Granulation,
[0057] Put the mixed powder prepared in the step (1) in the wet granulator, add the coloring agent of the prescribed amount into the wet granulator, and then add the solution in the step (2), and flow it after the addition of the solution is completed. Fluidized bed drying, control the drying temperature in the fluidized bed at 40°C to 80°C, until the moisture in the material is below 4%, take it out for later use;
[0058] (4) Tablets,
[0059] The granules prepared in the step (3) are added with a prescribed amount of lubricant, and then co...
Embodiment 1
[0062] Prescription composition (dosage per 1000 tablets, unit: g)
[0063] Raw materials Dosage (g) carbidopa 54.0 Levodopa 200.0 Hydroxypropyl Methyl Cellulose Solution 1 Hydroxypropyl Cellulose 20.0 Yellow Iron Oxide 1.0 Magnesium stearate 3.0 Co-made 1000 pieces
[0064] Weigh hydroxypropyl methylcellulose according to the prescription amount, add appropriate amount of hot water to make it swell, add purified water after cooling to make a solution for later use; weigh the prescription amount of carbidopa, levodopa, and hydroxypropyl cellulose Mix evenly, add yellow iron oxide and the above-mentioned binder for wet granulation machine granulation, fluidized bed drying, add additional magnesium stearate, mix evenly, and tablet with a conventional tablet machine.
Embodiment 2
[0066] Prescription composition (dosage per 1000 tablets, unit: g)
[0067]
[0068]
[0069] Weigh hydroxypropyl methylcellulose according to the prescription amount, add appropriate amount of hot water to make it swell, add purified water after cooling to make a solution for later use; weigh the prescription amount of carbidopa, levodopa, and hydroxypropyl cellulose Mix evenly, add yellow iron oxide and the above-mentioned binder for wet granulation machine granulation, fluidized bed drying, add additional magnesium stearate, mix evenly, and tablet with a conventional tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com