Cationic N-substituted aniline ionic liquid, polyionic liquid thereof, preparation method and application
A polyionic liquid, cationic technology, applied in the direction of organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
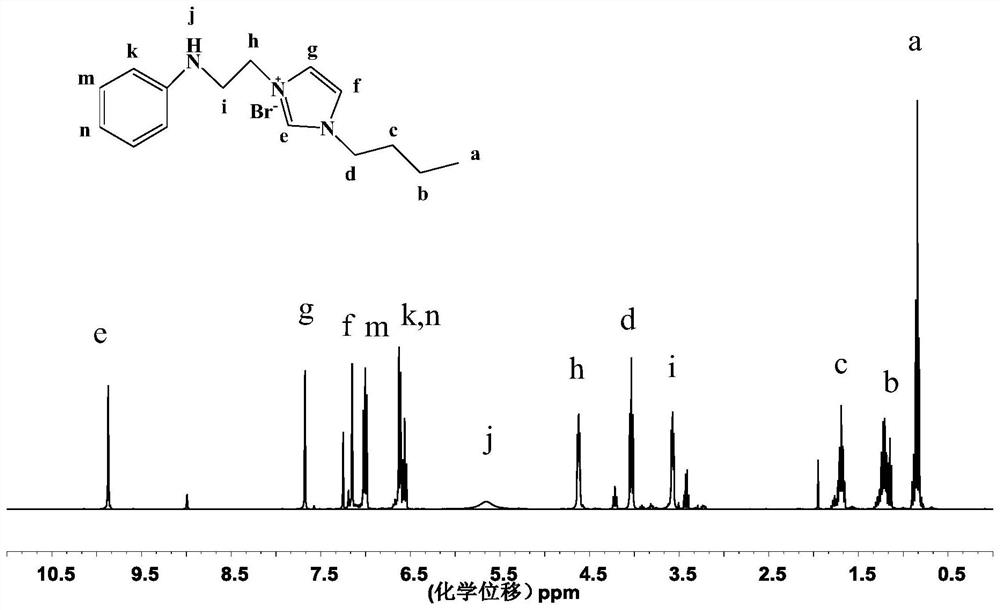
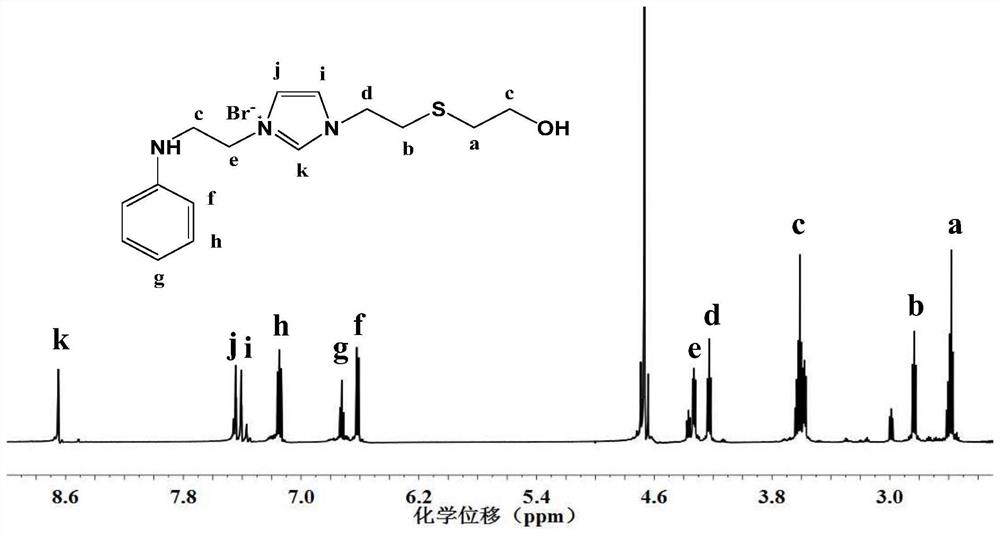
[0063] According to a preferred embodiment of the present invention, the cationic N-substituted aniline ionic liquid is selected from 1-(2'-anilino)ethyl-3-methylimidazolium bromide, 1,3-bis(2'-anilino) Ethyl imidazolium bromide, 1-(2'-anilino) ethyl-3-butyl imidazolium bromide (abbreviated as [AnEBIM]Br), 1-(2'-anilino) ethyl-3-vinyl Imidazolium bromide, 1-(2'-anilino)ethylpyridinium bromide, 1-(2'-anilino)ethyl-3-dodecyl imidazolium bromide (abbreviated as [AnEC 12 IM]Br), 1-anilinoethyl-3-[3'-(2"-hydroxyethyl)]ethanethiol imidazolium bromide (abbreviated as AEHETEI) or one or more.
[0064] According to the present invention, the cationic N-substituted aniline ionic liquid among the present invention is prepared by the method comprising the following steps:
[0065] Step 1, using N-phenylethanolamine to react with hydrobromic acid to obtain N-phenylethanolamine hydrobromide;
[0066] Step 2, reacting the N-phenylethanolamine hydrobromide obtained in step 1 with phosphorus...
Embodiment 1
[0145] Example 1 1-(2'-anilino)ethyl-3-butylimidazolium bromide ([AnEBIM]Br)
[0146] Add 6.8780g of N-phenylethanolamine to a 100mL round bottom flask, add 8.1355g of hydrobromic acid at 0°C, stir in an ice-water bath for 30min, and then stir at room temperature for 30min. Remove water by rotary evaporation, add chloroform to dissolve and dry with anhydrous sodium sulfate, filter, and remove solvent by rotary evaporation to obtain light yellow N-phenylethanolamine hydrobromide solid. The yield is 86.3%;
[0147]Add 4.68mmol of N-phenylethanolamine hydrobromide into the flask, add 5 mL of chloroform to dissolve it, blow argon to remove oxygen, slowly drop 0.6mL of phosphorus tribromide into the flask, and stir in a water bath at 40°C for 5h. Add 5 mL of deionized water to quench the reaction, extract 3 times with chloroform, and rotary evaporate the organic phase to obtain white solid N-phenylbromoethylamine hydrochloride with a yield of 62.9%;
[0148] Dissolve N-phenylbrom...
Embodiment 2
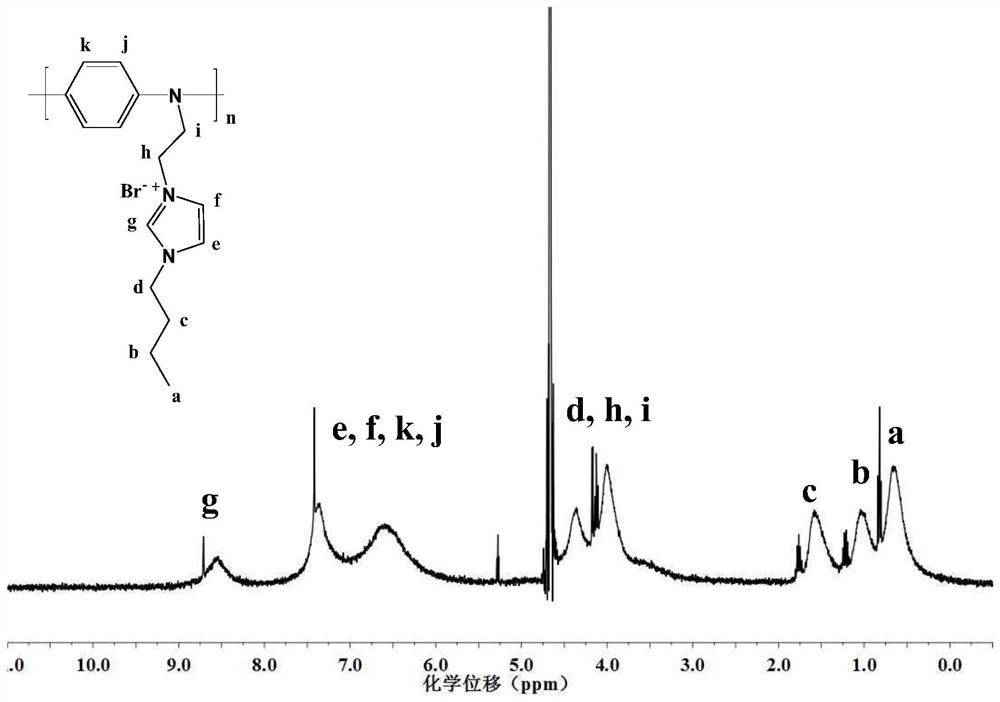
[0151] Example 2 1-(2'-anilino) ethyl-3-dodecyl imidazolium bromide ([AnEC 12 IM] Br)
[0152] The preparation process of Example 1 was repeated except that 0.3078g of 1-butylimidazole was replaced by 0.5858g of dodecylimidazole, and magnetically stirred in an oil bath at 120°C for 6 hours to obtain the product with a yield of 37.89%. Through nuclear magnetic spectrogram result, prove that gained product is 1-(2'-anilino) ethyl-3-dodecyl imidazolium bromide ([AnEC 12 IM] Br).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com