Application of exogenous ATG10S protein in preparation of antiviral drugs
An ATG10S, protein technology, applied in the field of medicine and biology, can solve problems such as no ATG10S research report, and achieve the effect of activating autophagy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
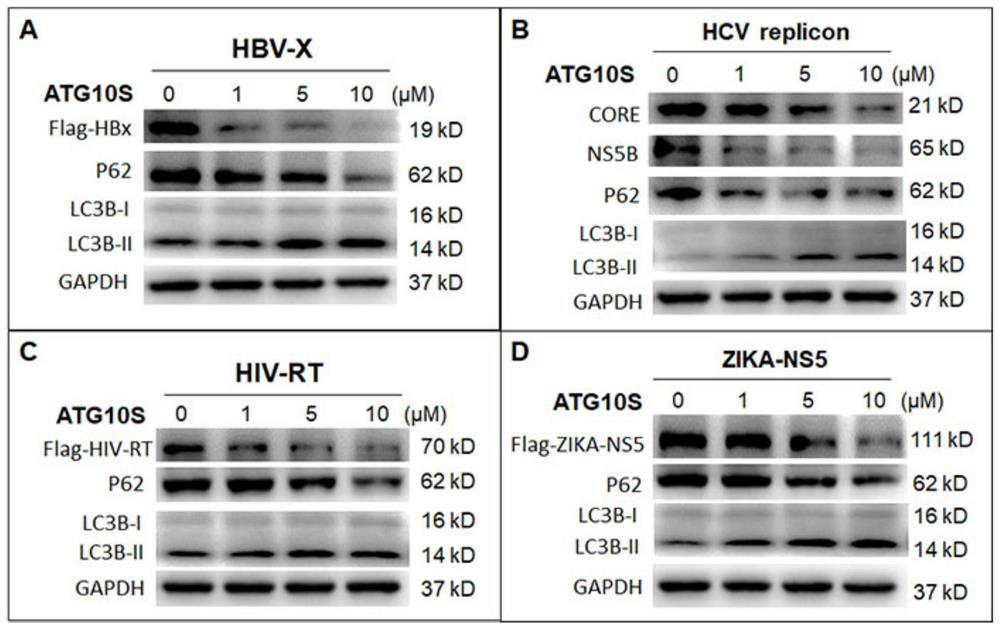
[0106] Example 1. The rhATG10S protein expressed in Escherichia coli degrades viral proteins by activating the autophagy activity of cells
[0107] The inventor's previous research has proved that the endogenous ATG10S protein of cells can degrade HCV viral proteins by promoting the formation of autophagy lysosomes 2-4 .
[0108] In the present invention, we examined whether the rhATG10S protein heterologously expressed in E. coli also functions as the endogenous protein.
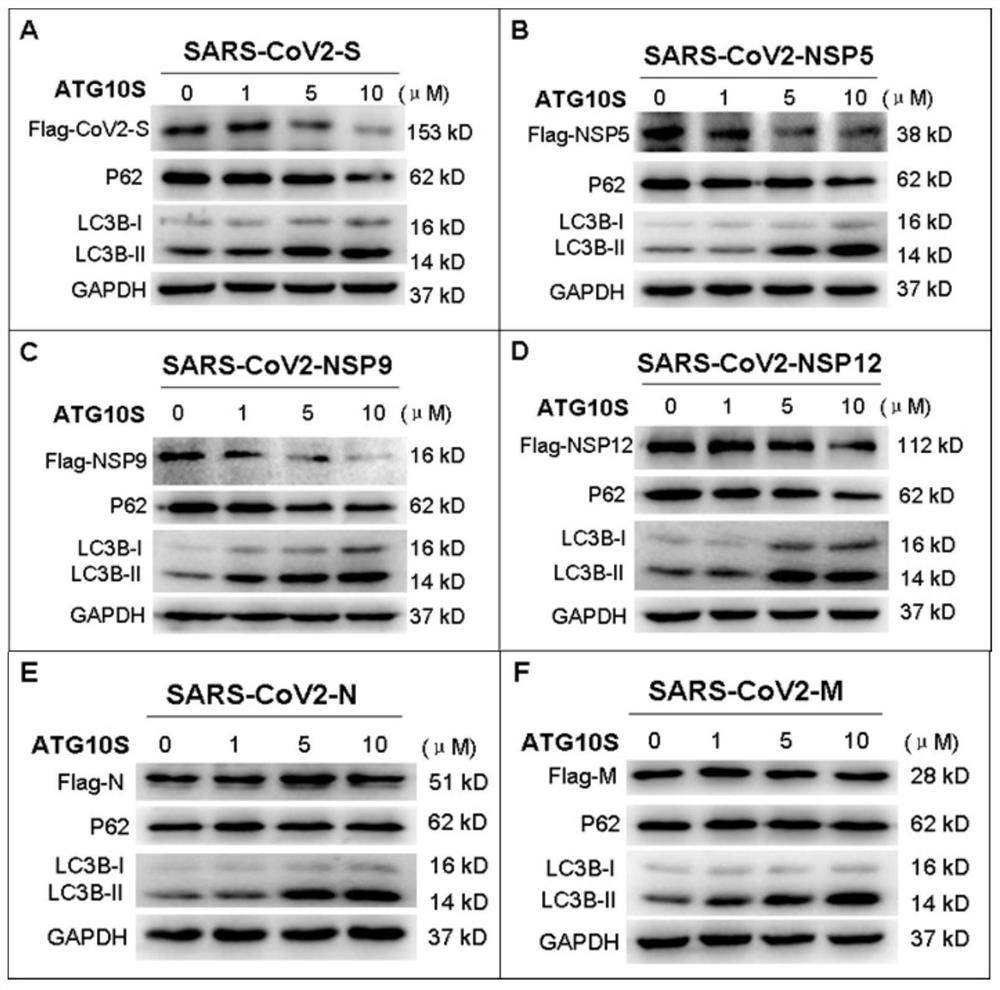
[0109] In the experiment, the inventors tested cells containing 11 viral proteins of 5 viruses (HBV-X, HCV-CORE / NS5B, HIV-RT, ZIKA-NS5, SARS-CoV2-S / N / M / NSP5 / NSP9 / NSP12) (each HepG2 cell transfers an exogenous protein, and 11 kinds of HepG2 cells transformed with different exogenous viral proteins were tested.) The rhATG10S protein expressed by Escherichia coli was added in the culture medium, and the viral protein and Levels of autophagic flux-associated proteins.
[0110] The results showed that after ...
Embodiment 2
[0112] Example 2, ATG10S expressed in Escherichia coli can enter the nucleus to activate the expression of type III interferon family genes
[0113] The inventor's previous research has confirmed that:
[0114] (1) In cells containing HCV, endogenous ATG10S protein can enter the nucleus as a new transcription factor, activate the promoter of type III interferon-2 (IFNL2) gene, and promote the high expression of IFNL2 2 ;
[0115] (2) IFNL2 protein can directly recognize and promote the fusion of autophagosomes and lysosomes, and enhance the activity of autophagy to degrade viral proteins;
[0116] (3) Down-regulation of IFNL2 can prevent the formation of autolysosomes and viral degradation 2,4 .
[0117] In order to further understand the anti-virus mechanism of rhATG10S derived from heterologous cells, in the present invention, we detected whether rhATG10S protein can also enter cells and related subcellular localization regions from extracellular.
[0118] 1. Add the rhA...
Embodiment 3
[0121] Embodiment 3, ATG10S antiviral mechanism
[0122] In order to further understand the antiviral mechanism of ATG10S, we tested the interaction between ATG10S and IFNL2 protein and autophagy flux protein by Co-IP experiment based on HCV subreplicon cell model. The results show,
[0123] 1. Compared with the model group and model group + ATG10, ATG10S can significantly enhance autophagic flux and increase IFNL2 protein levels; when immunoprecipitated with LAMP2 (a lysosomal membrane protein) antibody, it was found that ATG10S promoted lysosome Binding to IFNL2, P62, and LC3B (autophagophore marker), ATG10S was found to promote its binding to lysosomes and IFNL2 proteins when immunoprecipitated with antibodies to flag (here, flag-ATG10 and flag-ATG10S proteins) . But ATG10 has no such function ( Figure 8 ) 1 .
[0124] 2. Using the HCV whole virion infection experiment, the anti-whole virus effect of IFNL2 was detected. Taking non-infected cells as the control, the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com