Method for selectively preparing 5-hydroxymethylfurfural by catalyzing carbohydrates in water-ionic liquid mixed system
A technology of carbohydrates and hydroxymethylfurfural, which is applied in the direction of organic chemistry, can solve the problems of high price, unfavorable industrial production of 5-hydroxymethylfurfural, and increased production costs, and achieve low cost, reduction of levulinic acid and humin generation, the effect of reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: A method for selectively preparing 5-hydroxymethylfurfural from carbohydrates in a water-ionic liquid mixed system
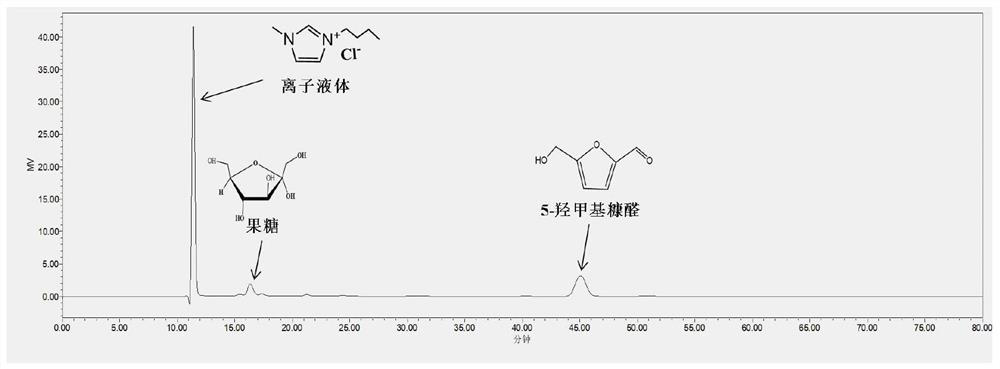
[0016] In the case of maintaining the same amount of fructose, ferric sulfate and deionized water as in Comparative Example 1, 15 wt% of 1-butyl-3-methylimidazolium chloride salt ionic liquid was added to the reaction system, and compared with Comparative Example 1 Under the same reaction conditions, the reaction solution containing the target product 5-hydroxymethylfurfural can be obtained. A small amount of the reaction solution is diluted and tested for detection. After quantitative analysis by high performance liquid chromatography, the obtained 5-hydroxymethylfurfural is calculated. The yield of furfural was 54.9%, the yield of by-product levulinic acid was 2.1%, and the yield of solid humin was 7.3%.
[0017] It can be seen from Example 1 and Comparative Example 1 that the present invention takes biomass-based carbohydrate as reactant, me...
Embodiment 2
[0020] Example 2: A method for selectively preparing 5-hydroxymethylfurfural from carbohydrates in a water-ionic liquid mixed system
[0021] In the case of maintaining the same amount of fructose, aluminum sulfate and deionized water as in Comparative Example 2, 15 wt% of 1-butyl-3-methylimidazolium chloride salt ionic liquid was added to the reaction system, and compared with Comparative Example 2 Under the same reaction conditions, the reaction solution containing the target product 5-hydroxymethylfurfural can be obtained. A small amount of the reaction solution is diluted and tested for detection. After quantitative analysis by high performance liquid chromatography, the obtained 5-hydroxymethylfurfural is calculated. The yield of furfural was 24.6%.
Embodiment 3
[0024] Example 3: A method for selectively preparing 5-hydroxymethylfurfural from carbohydrates in a water-ionic liquid mixed system
[0025] In the case of maintaining the same amount of fructose, copper sulfate and deionized water as in Comparative Example 3, 15 wt% of 1-butyl-3-methylimidazolium chloride salt ionic liquid was added to the reaction system, and compared with Comparative Example 3 Under the same reaction conditions, the reaction solution containing the target product 5-hydroxymethylfurfural can be obtained. A small amount of the reaction solution is diluted and tested for detection. After quantitative analysis by high performance liquid chromatography, the obtained 5-hydroxymethylfurfural is calculated. The yield of furfural was 43.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com