Styrene phosphonic acid curing agent, synthesis method and application
A technology of styrene phosphonic acid and synthesis method, which is applied in the fields of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as environmental pollution and high volatility of styrene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
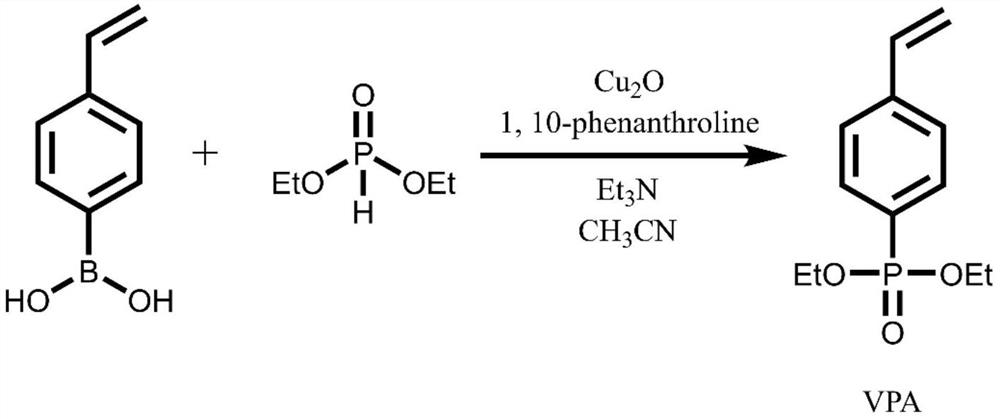
[0032] (1) The synthesis of 4-diethyl styrene phosphonate (VPA), its synthetic route is as follows:
[0033]
[0034] Take 2.96g of 4-vinylphenylboronic acid, 0.07g of cuprous oxide, 0.18g of phenanthroline, and 4.16mL of triethylamine into the reaction vessel, add 20mL of acetonitrile, and then add 1.38 g diethyl phosphonite. Open the mouth of the bottle and let it react at room temperature for 24 hours. After the reaction, the filtrate was rotary evaporated and passed through the column with ethyl acetate:petroleum ether=1:1 to obtain orange-yellow oily VPA.
[0035] (2) Synthesis of 4-alpha-methylstyrene phosphonic acid diethyl ester (MVPA), its synthetic route is as follows:
[0036]
[0037] Take 3.24g of 4-α-methylvinylphenylboronic acid, 0.07g of cuprous oxide, 0.18g of phenanthroline, and 4.16mL of triethylamine into the reaction vessel, add 20mL of acetonitrile, and wait for 4-α-methylethylene After the complete dissolution of phenylboronic acid, 1.38 g of di...
Embodiment 2-3
[0041] Steps (1), (2), and (4) in Examples 2 and 3 are the same as in Example 1, only the ratio of VPA to MVPA in step (3) is replaced by 3:1, 5:1 in turn.
Embodiment 4-5
[0043] Steps (1), (2), (4) in embodiment 4 and 5 are the same as in embodiment 1, only the ratio of styrene dilution in step (3) is replaced successively by 70%, 30%, that is Styrene accounts for 30% and 70% by mole of the styrene phosphonic acid curing agent composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com