Application of siRNA sequence in preparation of medicine for treating ovarian cancer
A DNA sequence, ovarian cancer technology, applied in the direction of retroRNA virus, DNA / RNA fragments, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment discloses a method for preparing a recombinant vector, comprising:
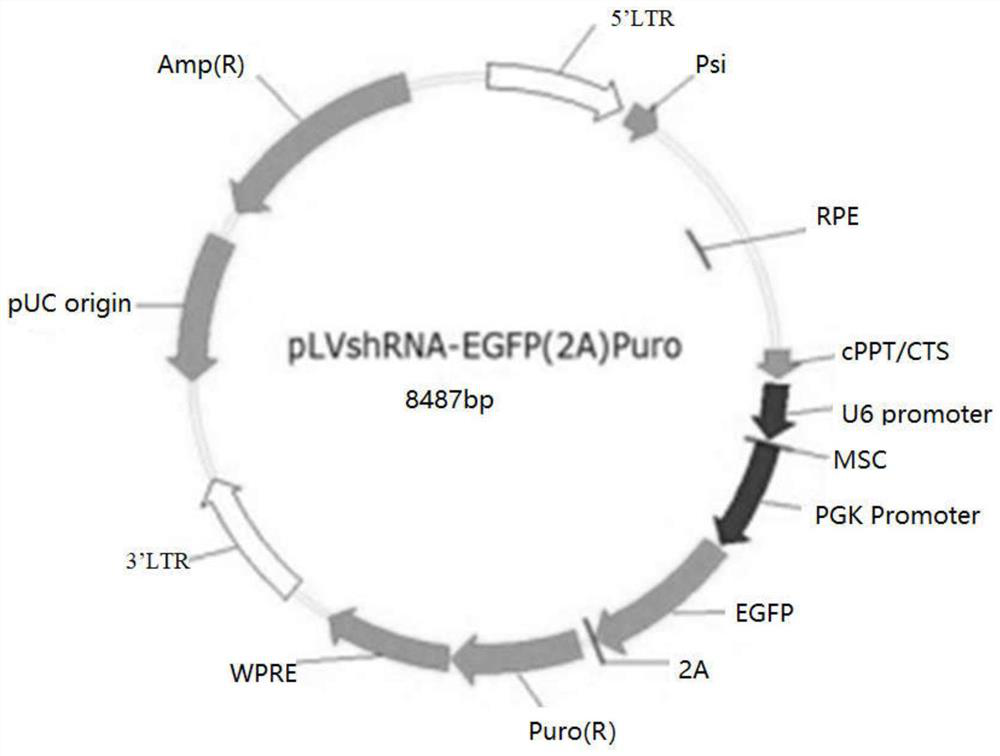
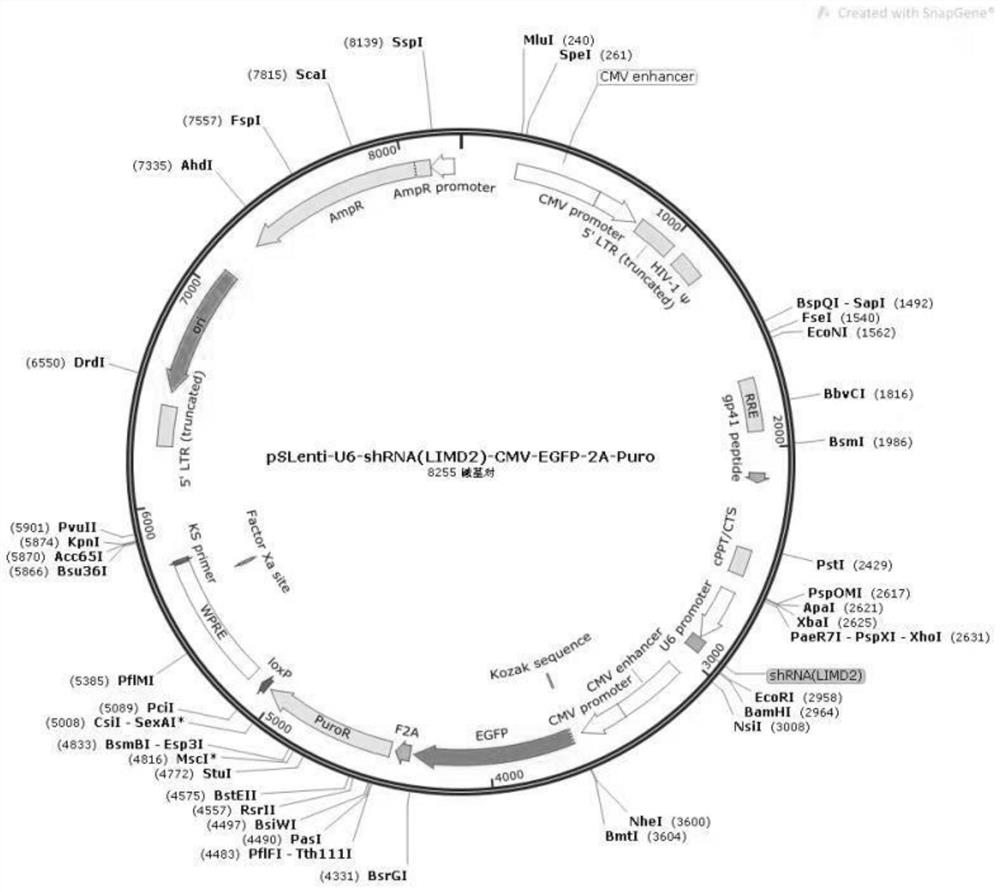
[0029] Firstly, the DNA sequence group (forward sequence: 5'-GACCCACCCAACTTACCATA-3'(SEQ ID NO: 1); reverse sequence: 5'- AATTCTCCGAACGTGTCAC-3' (SEQ ID NO: 2)) was cloned into the lentiviral vector pLVshRNA-EGFP(2A) Puro vector to obtain pSLenti-U6-shRNA(LIMD2)-CMV-EGFP-2A-Puro vector. Then, use the helper plasmid to package the lentivirus. Next, use the packaged lentivirus to infect ovarian cancer cells A2780, and 24 hours later, use DMEM culture medium containing 5 μg / ml of puromycin to screen for 48 hours, and the obtained ovarian cancer cells after transfection are subjected to cell migration and subcutaneous Tumor formation experiments.
[0030] The schematic diagram of the pLVshRNA-EGFP (2A) Puro vector and the schematic diagram of the pSLenti-U6-shRNA (LIMD2)-CMV-EGFP-2A-Puro vector are respectively as follows figure 1 and figure 2 shown.
Embodiment 2
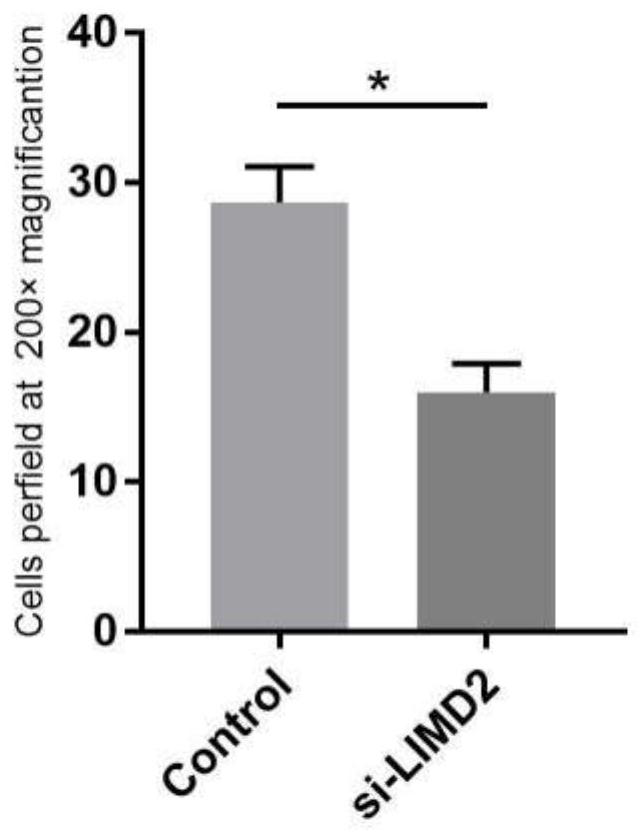
[0032] Cell migration is an important indicator of tumor metastasis, and Transwell can be used to study the motility of tumor cells.
[0033] There is a permeable polycarbonate membrane on the bottom of the Transwell chamber, the upper chamber is a serum-free cell suspension, and the lower chamber is a serum-containing medium. Certain components in the serum, such as some cytokines, are essential for cell growth , so the cells have tropism for these factors, and the cell tropism is used to promote the transfer of cells to the lower chamber, and different cells will show different motility transfer capabilities here.
[0034] The transwell chamber in the Corning transfer assay kit is composed of a 24-well tissue culture plate and a 12-well cell culture insert. The insert contains a polycarbonate membrane with a pore size of 8 μm. The transferred cells migrate and adhere to the bottom of the polycarbonate membrane.
[0035] This example studies the effect of siRNA on the migrati...
Embodiment 3
[0044] This embodiment relates to nude mouse tumor formation experiments, specifically comprising the following steps:
[0045] 6-week-old female mice were subcutaneously injected with 200 microliters (3×10 6) cell mixture (100 microliters cell PBS+100 microliters Matrigel). Tumor size was measured every 3 days. Tumor volume was calculated according to the following formula: V=a×b 2 / 2. After 3 weeks, the nude mice were euthanized by intraperitoneal injection of 2% pentobarbital sodium (50 mg / kg), and the tumor body was weighed. Part of the tissue was fixed with 10% formaldehyde for subsequent histological testing; the remaining tissue was quickly frozen in liquid nitrogen and stored in a -70°C refrigerator for subsequent molecular biological testing. The injected cell density was 3 x 10 7 / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com