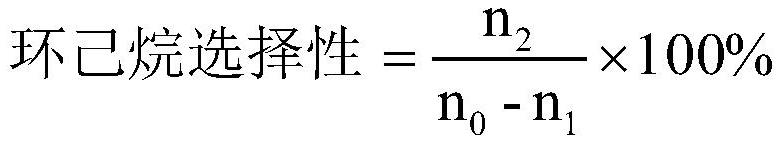Method for synthesizing cyclohexane through 2,6-dimethoxyphenol
A technology of dimethoxyphenol and cyclohexane, applied in chemical instruments and methods, producing hydrocarbons from oxygen-containing organic compounds, carbon compound catalysts, etc., to achieve good reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of NiO or Pt / NiO catalyst by deposition precipitation method: prepare a solution with a nickel nitrate concentration of 0.5mol / L and a platinum chloride concentration of 0.005mol / L, and add it dropwise to a 1mol / L NaOH solution while stirring, and Stir at room temperature for 2 h, then filter and wash the filter cake with deionized water until the filtrate is neutral. The filter cake was dried at 380K for 12h, and finally the catalyst was calcined in a muffle furnace at 623K for 2h, dried and sealed for future use.
[0028] Take 0.05g of the catalyst and put it in the high-pressure batch reactor, and then add 0.50g of 2,6-dimethoxyphenol and 10ml of n-decane into the high-pressure batch reactor. The reactor was sealed, purged with nitrogen for 3 times, and then 3.0 MPa hydrogen was introduced. The temperature of the reactor was raised to 573K, and the stirring rate was 700rpm to continue the reaction for 2h.
Embodiment 2
[0030] The reaction time was 4h, and the catalyst preparation method, feed amount and reaction conditions were basically the same as in Example 1.
Embodiment 3
[0032] The reaction temperature is 623K, and the catalyst preparation method, feed amount and reaction conditions are basically the same as in Example 1.
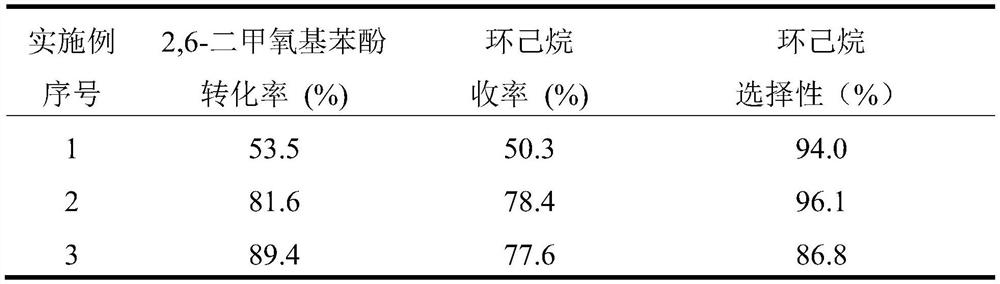
[0033] The reaction result of embodiment 1~3 is shown in table 1.
[0034] Table 1. Embodiment 1~3 reaction result summary table
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com