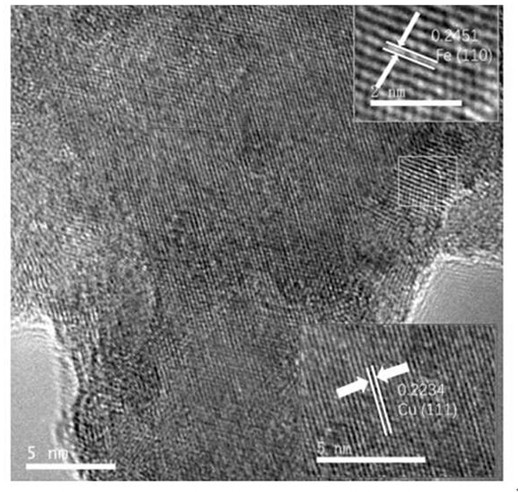
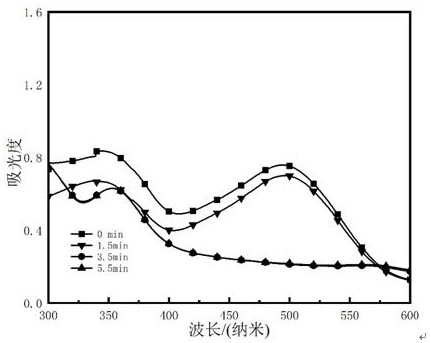
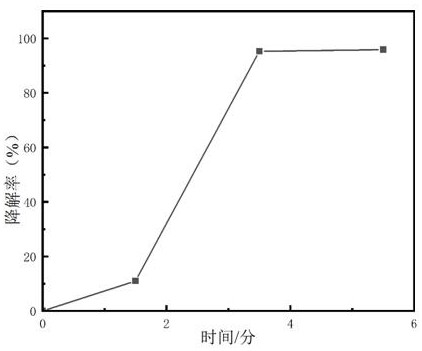
Cu-Fe bimetallic nanosheet and preparation method thereof
A bimetallic nano, cu-fe technology, applied in metal processing equipment, nanotechnology, nanotechnology, etc., can solve the problems of high preparation temperature, single surface morphology, and limited development of nano-copper-iron materials, and achieve good repeatability The effect of utilization performance, simple preparation method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Cu 0.9 Fe 0.1 bimetallic nanosheets
[0031] 1) Prepare 10 mol / L NaOH solution: Dissolve 100g of NaOH in a beaker with deionized water, transfer the solution to a 250mL volumetric flask after cooling, wash, constant volume and shake well before use;
[0032] 2) Prepare 0.5 mol / L CuSO 4 solution, 0.5 mol / L FeCl 3 Solution: 7.98g of CuSO 4 , 8.11g of FeCl 3 Dissolve in different beakers with deionized water, transfer to different 100mL volumetric flasks after complete dissolution, wash, constant volume and shake well before use;
[0033] 3) Take 200mL of the NaOH solution prepared in step 1) in a flask, and put the CuSO prepared in step 2) 4 solution and FeCl 3 Add 9mL and 1mL of the solution into the flask respectively, then add 16.7 mL of anhydrous ethylenediamine and 5 mL of 1 mol / L ascorbic acid solution, mix them evenly and heat in a water bath at 60°C for 0.5h;
[0034] 4) After step 3) the water bath heating is completed, the reaction product...
Embodiment 2
[0036] Preparation of Cu 0.8 Fe 0.2 bimetallic nanosheets
[0037] 1) Prepare a NaOH solution with a substance concentration of 15mol / L: Dissolve 150g of NaOH in a beaker with deionized water, transfer the solution to a 250mL volumetric flask after cooling, wash, constant volume and shake well before use;
[0038] 2) The concentration of the prepared substance is 0.2 mol / L CuCl 2 solution, 0.2 mol / L Fe(NO 3 ) 3 Solution: 2.69g of CuCl 2 , 4.84g of Fe(NO 3 ) 3 Dissolve in different beakers with deionized water, transfer to different 100mL volumetric flasks after complete dissolution, wash, constant volume and shake well before use;
[0039] 3) Take 200mL of the NaOH solution prepared in step 1) in a flask, and take the CuCl prepared in step 2) 2 solution, Fe(NO 3 ) 3 Add 8 mL and 2 mL of the solution into the flask respectively, then add 3.8 mL of anhydrous ethylenediamine and 6 mL of 1 mol / L glucose solution, shake them well and heat in a water bath at 70°C for 1 h; ...
Embodiment 3
[0042] Preparation of Cu 0.7 Fe 0.3 bimetallic nanosheets
[0043] 1) Prepare 20mol / L NaOH solution: Dissolve 200g of NaOH in a beaker with deionized water, transfer the solution to a 250mL volumetric flask after cooling, wash, constant volume and shake well before use;
[0044] 2) Prepare 0.2mol / L CuCl 2 solution, 0.2mol / L Fe(NO 3 ) 3 Solution: 2.69g of CuCl 2 , 4.84 Fe(NO 3 ) 3 Dissolve in different beakers with deionized water, transfer to different 100mL volumetric flasks after complete dissolution, wash, constant volume and shake well before use;
[0045] 3) Take 200mL of the NaOH solution prepared in step 1) in a flask, and put the CuCl prepared in step 2) 2 solution and Fe(NO 3 ) 3 Add 7mL and 3mL of the solution into the flask respectively, then add 2.7mL of anhydrous ethylenediamine and 0.61 mL of hydrazine hydrate solution with a mass fraction of 80%, mix them uniformly and heat in a water bath at 80°C for 1.5h;
[0046] 4) After step 3) the water bath hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com