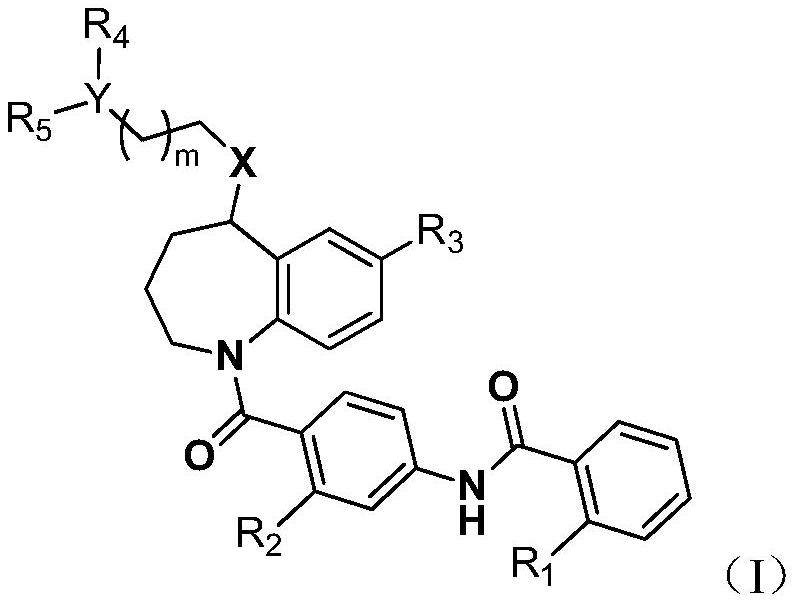
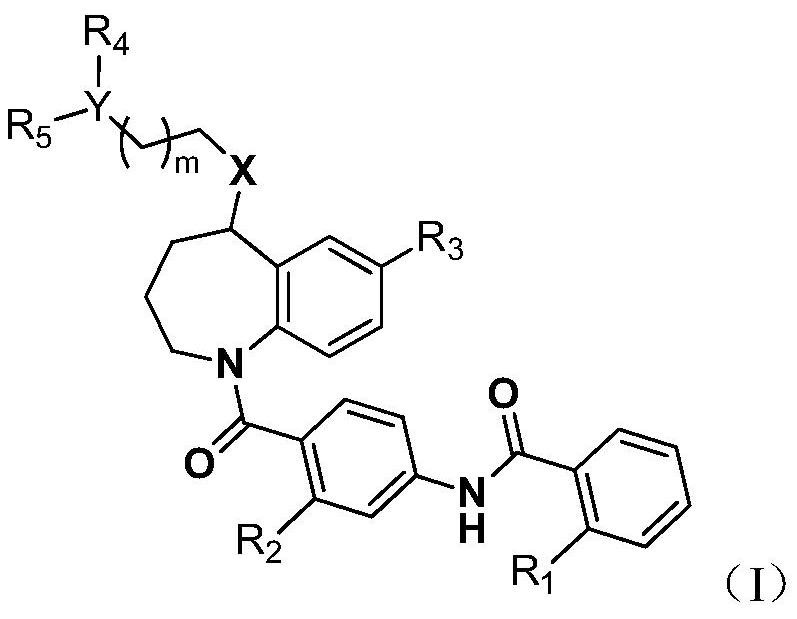
Novel benzoazepine compound, composition and applications of novel benzoazepine compound and composition
A compound and heterocyclic group technology, applied in the field of new benzazepine compounds, can solve the problems of insufficient activity, side effects and physicochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
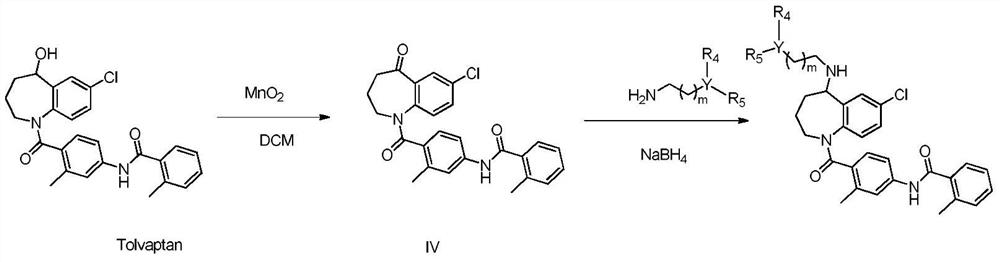
[0137] N-(4-(7-chloro-5-((3-morpholinopropyl)amino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine Preparation of -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (compound 1)
[0138] Step 1: N-(4-(7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepine - Preparation of -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (intermediate IV):
[0139]
[0140] Dissolve tolvaptan (1.0 g, 2.23 mmol) in dichloromethane (30 mL), add active manganese dioxide (1.0 g), and stir under reflux overnight. The solvent was evaporated to dryness under reduced pressure, and the solid residue was purified by silica gel column chromatography (eluent: 100% EA) to obtain light yellow oily intermediate IV (0.90 g, yield: 90.4%)
[0141] HRMS (ESI) calculated for C 26 h 24 Cl N 2 o 3 + [M+H] + 447.1470; Found: 447.1473
[0142] Step 2: N-(4-(7-chloro-5-((3-morpholinopropyl)amino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (compound 1) preparation:
[0143] ...
Embodiment 2
[0147] N-(4-(7-chloro-5-((4-morpholinobutyl)amino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine Preparation of -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (compound 2)
[0148] Step 1: Preparation of 4-morpholinobutyronitrile (intermediate V):
[0149]
[0150] 4-Chlorobutyronitrile (0.90 g, 8.69 mmol) was dissolved in morpholine (4.0 mL), and stirred overnight at room temperature. Silica gel column chromatography (eluent: MeOH / DCM = 1:20) separated intermediate V (0.85 g, yield: 63%) as a pale yellow transparent liquid. 1 H NMR (800MHz, CDCl 3 )δ3.75–3.65(m,4H),2.54–2.33(m,8H),1.87–1.78(m,2H).HRMS(ESI)calculated for C 8 h 15 N 2 o + [M+H] + 155.1179; Found: 155.1178.
[0151] Step 2: Preparation of 4-morpholino-1-butylamine (intermediate VI):
[0152]
[0153] Intermediate V (0.31 g, 2 mmol) was dissolved in methanol (10 mL), palladium / carbon catalyst (10% Pd, 300 mg) and concentrated hydrochloric acid (0.5 mL) were added. Stir at 45°C for 2 hours under hydrogen ...
Embodiment 3
[0158] N-(4-(7-chloro-5-((5-morpholinopentyl)amino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (compound 3) preparation:
[0159]
[0160] The 4-chlorobutyronitrile in Step 1 of Example 2 was replaced by 5-bromovaleronitrile, and the rest of the required raw materials, reagents and preparation methods were the same as in Example 2 to obtain white foamy compound (I-3). 1 H NMR (800MHz, CDCl 3 )δ7.70–6.45(m,10H),4.57–4.01(m,1H),3.87–3.64(m,5H),3.21–3.05(m,1H),2.71–2.39(m,14H),2.11– 1.97(m,2H),1.81–1.32(m,8H).HRMS(ESI)calculated for C 35 h 44 ClN 4 o 3 + [M+H] + 603.3096; Found: 603.3098.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com