Nano-drug taking irinotecan as carrier as well as preparation method and application of nano-drug
An irinotecan and nano-drug technology, applied in the field of medicine, can solve the problems of killing and inhibiting difficult tumor tissues, monomer degradation toxicity, slow onset of effect, etc., to achieve the treatment of metastatic colorectal cancer, reduce toxic side effects, increase dispersive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
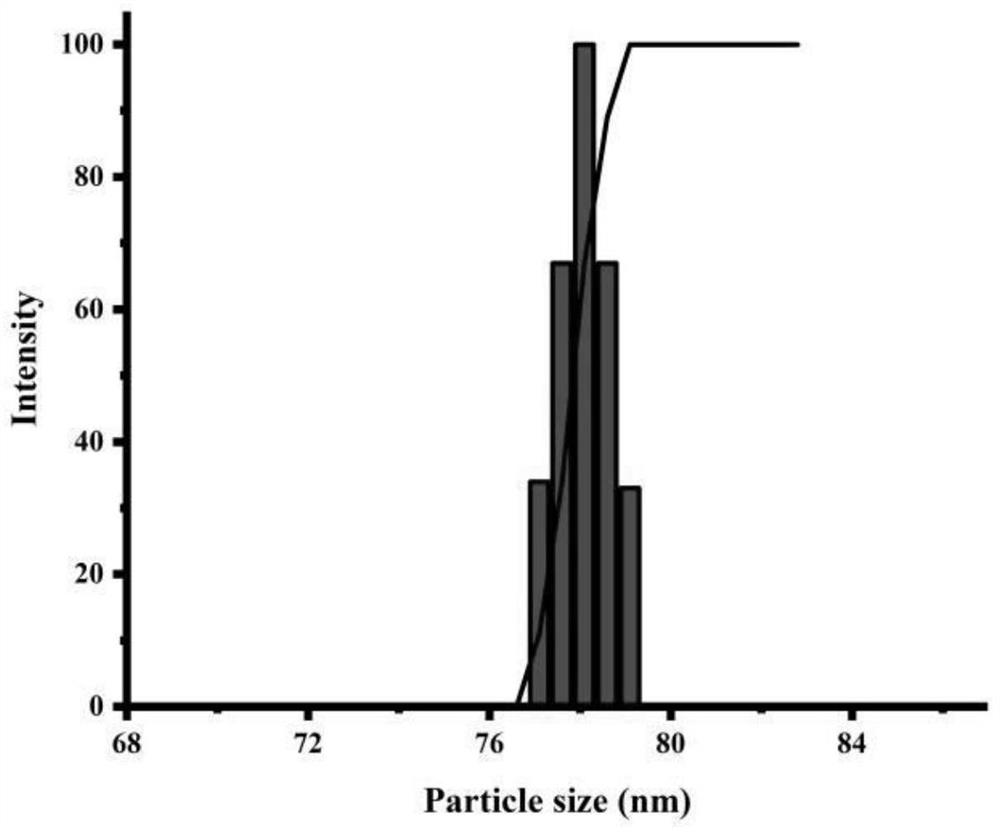
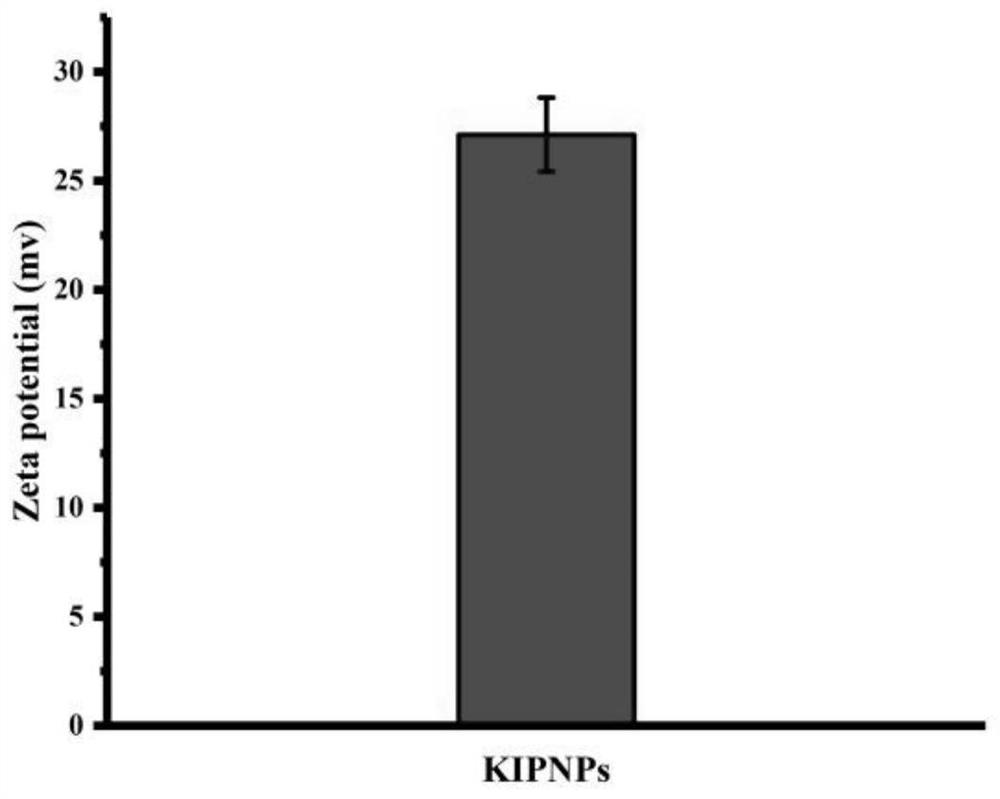
[0040]Example 1 According to the optimal mass ratio of irinotecan:carprofen at 2:1, three groups of 10 mg irinotecan were weighed and placed in three 1.5 ml EP tubes, and 200 μl DMSO was added to irinotecan 1.5 ml EP and ultrasonically weighed three groups of 5 mg carprofen respectively and dissolved them in the above solution to form a mixed solution. Slowly drop the mixed solution into 5 ml 0.5 mg / ml PVP aqueous solution, limit the dropping rate to 2 s / drop, stir at 500 r / min for 2.5 min, centrifuge at 2000-6000 r / min for 3-10 min, take The particle size and Zeta potential of the supernatant were measured, and finally figure 1 and figure 2 . The particle size of the obtained nanoparticles is about 78 nm, and the Zeta potential is about 27 mv.
Embodiment 2
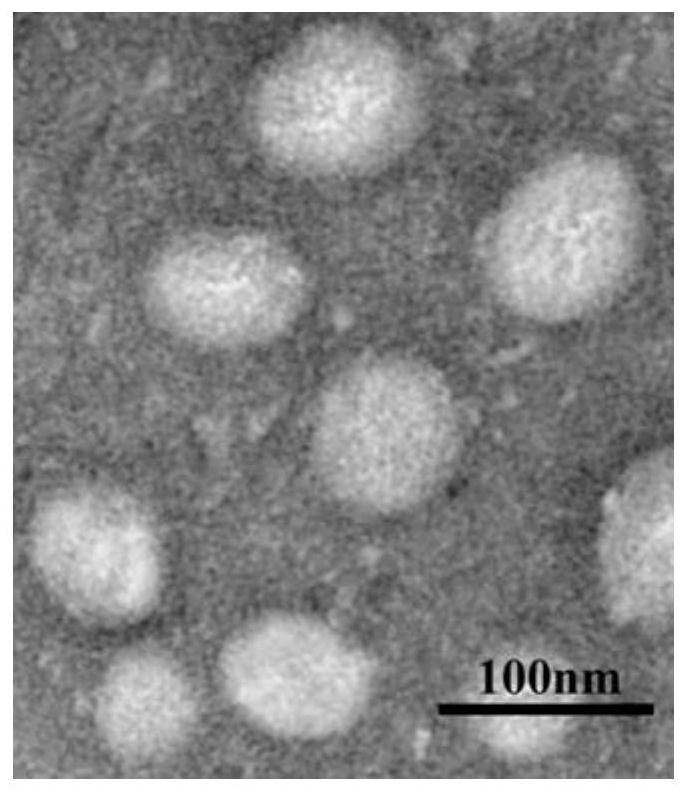
[0041] Example 2 According to the preparation method in Example 1, the obtained irinotecan and carprofen nanoparticles were prepared, and the solution was concentrated by gradient centrifugation, and 20 μl was dropped into the copper grid, and phosphotungstic acid was added to the Dry under infrared light for 5 min, and take transmission electron microscope pictures after the water evaporates, and finally get image 3 . The resulting particle size was around 73 nm.
Embodiment 3
[0042] Example 3 According to the optimal mass ratio of irinotecan: carprofen at 2:1, weigh three groups of 10 mg irinotecan and place them in three 1.5 ml EP tubes, add 200 μl DMSO to irinotecan 1.5 ml EP and ultrasonically weighed three groups of 5 mg carprofen respectively and dissolved them in the above solution to form a mixed solution. Slowly drop the mixture into 5 ml 0.5 mg / ml PVP aqueous solution, limit the dropping rate to 2 s / drop, stir at 500 r / min for 2.5 min, centrifuge at 2800 r / min for 5 min, and take the supernatant for 3.5 min ml to measure the particle size for 1 h. After measuring the particle size, the three groups of nanoparticles were placed in a refrigerator at 4 °C, and the particle size was measured at the same time every day. Three more groups were prepared according to the same method, and the particle size was measured and stored in -20 °C freezer. After thawing at room temperature, the particle size change was measured at the same time every day,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com