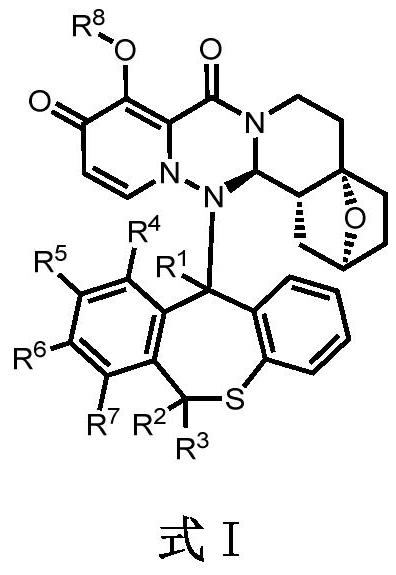
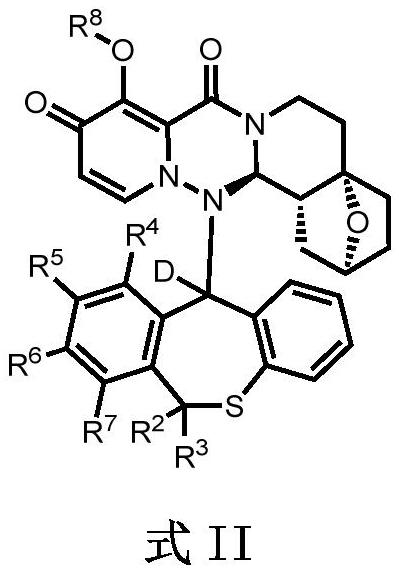
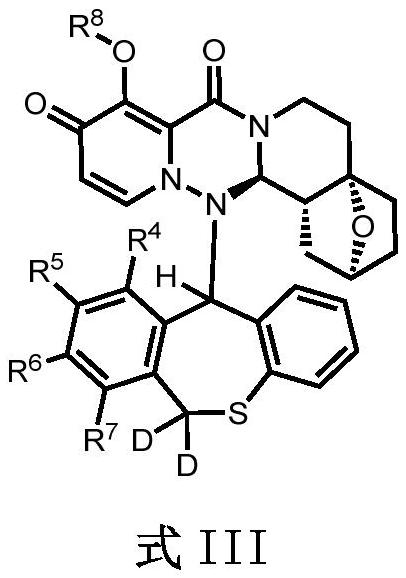
Condensed polycyclic pyridone derivative and application thereof
A technology of polycyclic pyridone and derivatives, applied in the directions of active ingredients of heterocyclic compounds, organic chemistry, antiviral agents, etc., can solve the problems of low immunity and low efficacy in young children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0191] (2S, 4aS, 14aR, 14bR)-14-((S)-10-fluoro-6-hydrogen-11-deuterodibenzo[b,e]thieheptin-11-yl)-9-hydroxyl-1 , 3,4,5,6,14,14a,14b-octahydro-2H-2,4a-epoxypyrido[1',2':1,6][1,2,4]triazino[ 3,4-a]isoquinoline-8,10-dione [compound 1] and (2S,4aS,14aR,14bR)-14-((R)-10-fluoro-6-hydrogen-11-deuterodione Benzo[b, e]thieheptin-11-yl)-9-hydroxyl-1,3,4,5,6,14,14a,14b-octahydro-2H-2,4a-epoxypyrido[ 1',2': 1,6][1,2,4]triazino[3,4-a]isoquinoline-8,10-dione [compound 2]
[0192] The synthetic route is as follows:
[0193]
[0194] Step 1: DMF (300 mL) intermediate 1-1 (50 g, 521 mmol) and nitromethane (32 g, 521 mmol) were added to a round bottom flask. NaOH (62.4 g, 1.56 mmol) was added at 0°C. Stirring of the resulting mixture was continued at room temperature for 2 hours. The reaction was quenched with water, then concentrated in vacuo. The residue was diluted with water and extracted with ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate and ...
Embodiment 3 and 4
[0210] (2S, 4aS, 14aR, 14bR)-14-((S)-10-fluoro-6,6-dideutero-11-hydrodibenzo[b,e]thiepin-11-yl)-9- Hydroxy-1,3,4,5,6,14,14a,14b-octahydro-2H-2,4a-epoxypyrido[1',2':1,6][1,2,4]tri Azino[3,4-a]isoquinoline-8,10-dione [compound 3] and (2S,4aS,14aR,14bR)-14-((R)-10-fluoro-6,6-di Deuterium-11-hydrodibenzo[b,e]thieheptin-11-yl)-9-hydroxy-1,3,4,5,6,14,14a,14b-octahydro-2H-2,4a -epoxypyrido[1',2':1,6][1,2,4]triazino[3,4-a]isoquinoline-8,10-dione [Compound 4]
[0211] The synthetic route is as follows:
[0212]
[0213] Step 1: Add Intermediate 1-14 (200 mg, 0.41 mmol) and Intermediate 3-1 (300 mg, 0.41 mmol) to a round bottom flask A stirred solution of T3P (1.8 mL) and EtOAc (2 mL) was added methanesulfonic acid ( 39 mg, 0.41 mmol). Stirred at 50 °C for 24 hours. Quenched the reaction with H2O. The resulting mixture was extracted with EtOAc, and the combined organic layers were dried over anhydrous Na2SO4. After filtration, the filtrate was concentrated under reduced pressure t...
Embodiment 5 and 6
[0216] (2S, 4aS, 14aR, 14bR)-14-((S)-10-fluoro-6,6-dideutero-11-deuterodibenzo[b,e]thiepin-11-yl)-9- Hydroxy-1,3,4,5,6,14,14a,14b-octahydro-2H-2,4a-epoxypyrido[1',2':1,6][1,2,4]tri Azino[3,4-a]isoquinoline-8,10-dione [compound 5] and (2S,4aS,14aR,14bR)-14-((R)-10-fluoro-6,6-di Deuterium-11-deuterodibenzo[b,e]thieheptin-11-yl)-9-hydroxy-1,3,4,5,6,14,14a,14b-octahydro-2H-2,4a -epoxypyrido[1',2':1,6][1,2,4]triazino[3,4-a]isoquinoline-8,10-dione [Compound 6]
[0217] The synthetic route is as follows:
[0218]
[0219] Step 1: Intermediate 1-14 (150 mg, 0.31 mmol) and Intermediate 5-1 (225 mg, 0.31 mmol) were added to a round bottom flask A stirred solution of T3P (1.35 mL) and EtOAc (1.5 mL) was added methanesulfonic acid (30 mg, 0.31 mmol). Stirred at 50°C for 24 hours. Quenched the reaction with H2O. The resulting mixture was extracted with EtOAc, and the combined organic layers were dried over anhydrous Na2SO4. After filtration, the filtrate was concentrated under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com