Combination therapy for the treatment of prostate cancer
A technology for prostate cancer, therapy, applied in the field of combination therapy for the treatment of prostate cancer, which can solve the problem of unpredictable overall profile, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1: the synthesis of compound I
[0168] Step A: 5-Bromo-N 3 Synthesis of -(phenylmethylene)pyridine-2,3-diamine (compound B)
[0169]
[0170] Starting material A was dissolved in methanol and acetic acid. The solution was cooled to 0°C to 5°C and benzaldehyde was added dropwise. Once the reaction is complete, add treated water and NaHCO dropwise 3 solution, kept at low temperature (0°C to 5°C). The solid was filtered off and washed with methanol / water (1:1) followed by drying to give compound B in 94% yield and +99% purity (by HPLC). 1 H-NMR (DMSO-d 6 ): δ8.75(1H), 8.04(2H), 7.93(1H), 7.65(1H), 7.50-7.60(3H).
[0171] Step B: N 3 -Synthesis of benzyl-5-bromopyridine-2,3-diamine (compound C)
[0172]
[0173] Compound B was dissolved in ethanol and NaHB was added portionwise 4 , keeping the temperature between 15°C and 25°C. The reaction mixture was stirred for 8 to 15 hours until completion as monitored by HPLC. The HCl solution was added to...
Embodiment 2
[0186] Example 2: Crystalline Mesylate Salt of Compound I
[0187] About 5 g of compound I was dissolved in ethanol (115 mL) and a solution of methanesulfonic acid in ethanol (10 mL, 158.7 mg / mL) was added according to a 1:1 molar ratio. The mixture was shaken at 50°C for 2 hours, then concentrated to half volume and stirred overnight. The solid formed (mesylate salt of compound I / co-crystal Form I) was isolated, dried and characterized.
[0188] The mesylate salt / co-crystal Form I of Compound I was also obtained from other solvents and solvent mixtures including acetone and acetonitrile.
[0189] The mesylate / co-crystal form I of Compound 1 was characterized by XRPD as comprising the following peaks in 2Θ: 8.4±0.2, 10.6±0.2, 11.7±0.2, 14.5±0.2, 15.3±0.2, 16.9±0.2, 18.2±0.2 0.2, 19.0±0.2, 19.9±0.2, 20.5±0.2, 22.6±0.2, 23.8±0.2, 24.5±0.2 and 27.6±0.2, such as using Cu-K α The radiation tube was measured on a diffractometer ( Figure 4 ).
[0190] The mesylate / co-crystal fo...
Embodiment 3
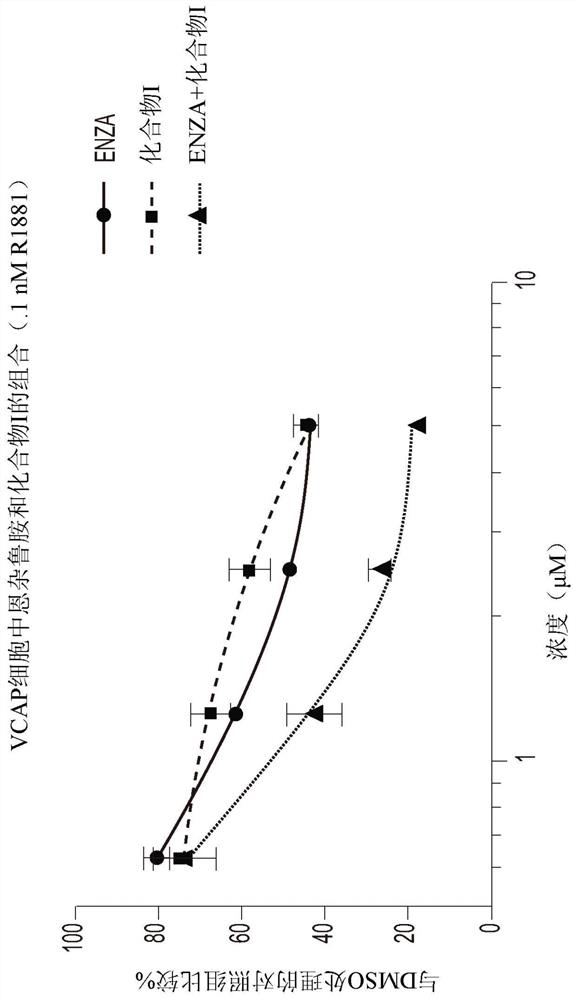
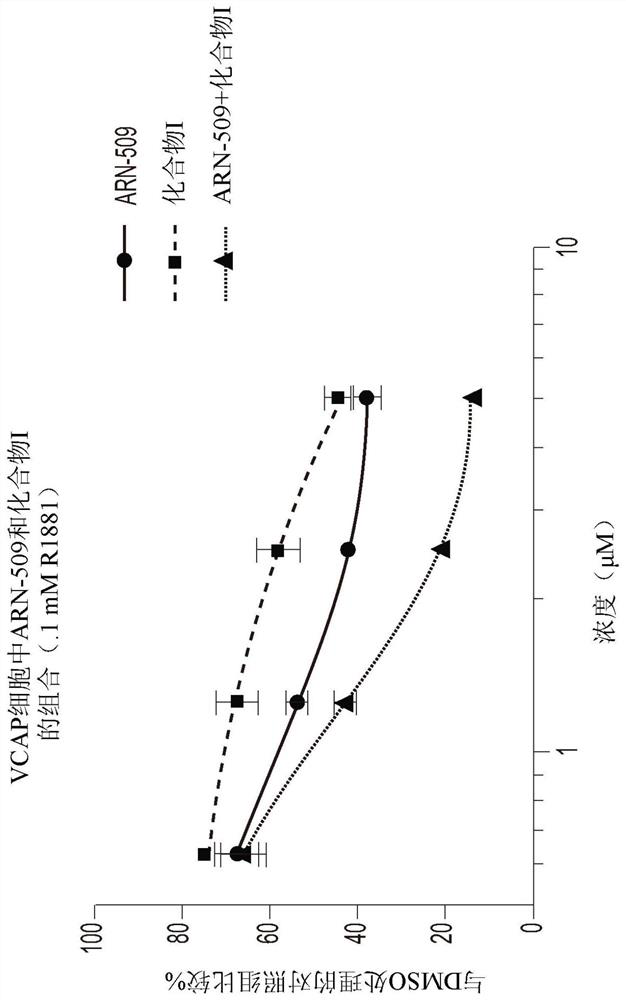
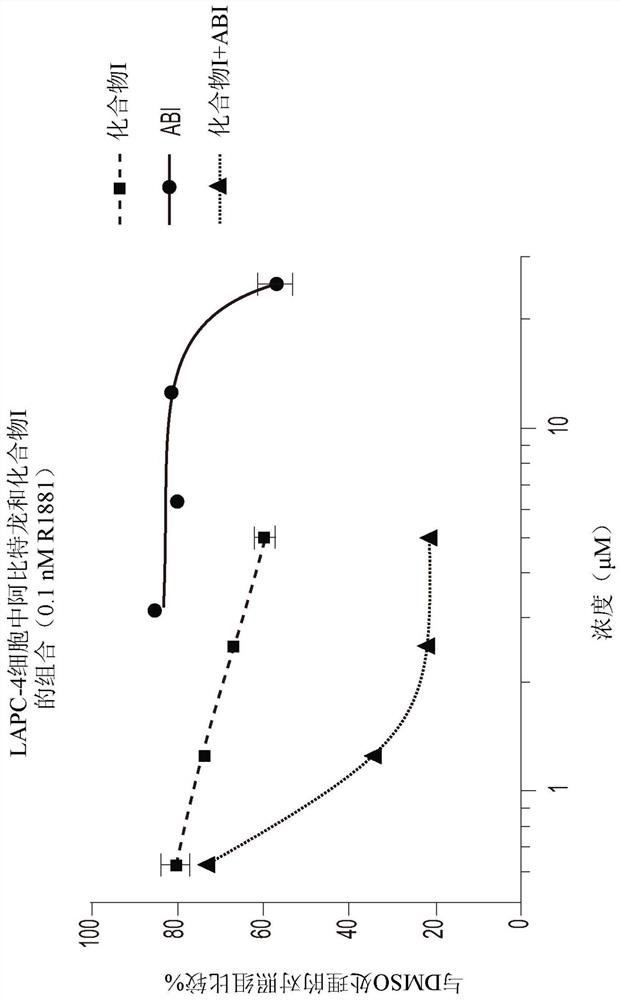
[0192] Example 3: Synergistic inhibition of VCaP cell viability by combining Compound I with Enzalutamide
[0193] VCaP cells (CRL-2876) were plated at a density of 10,000 cells per well on a 96-well flat-bottomed plate with D-MEM medium (containing 10% charcoal-removed FBS and penicillin / streptomycin), and incubated at 37°C for 5 %CO 2 Incubate for 24 hours. The medium was replaced with D-MEM and incubated at 37°C, 5% CO 2 For 3 to 7 days under incubation, the D-MEM contained 10% charcoal-depleted FBS, and had a constant ratio of Compound I or enzalutamide as a single agent or at four different concentrations (2×IC50, 1×IC50, 0.5×IC50, 0.25×IC50) of 0.1 nM R1881 treated with the combination of the two drugs. If cells were grown for 7 days, they were reprocessed on day 3 or 4 as described above. If cells were grown for 7 days, they were reprocessed on day 3 or 4 as described above. Triplicate wells were used for each concentration and wells containing only medium with 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com