Medical application of CREG1 protein in prevention or treatment of sorafenib-induced myocardial injury
A myocardial injury, protein technology, applied in the medical use of myocardial injury and related diseases, CREG1 protein or its active fragment field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
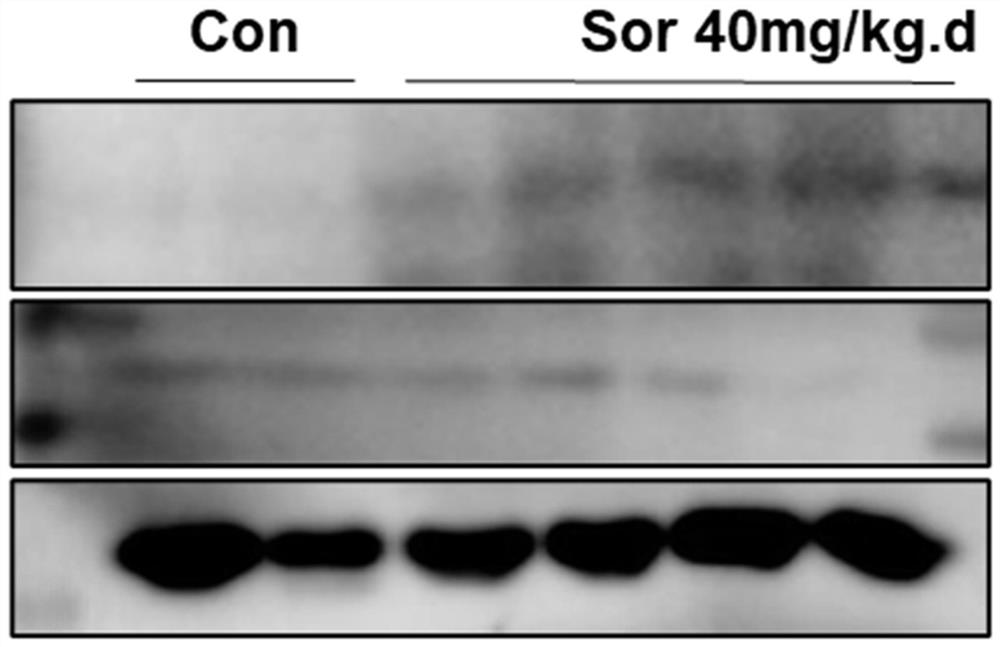
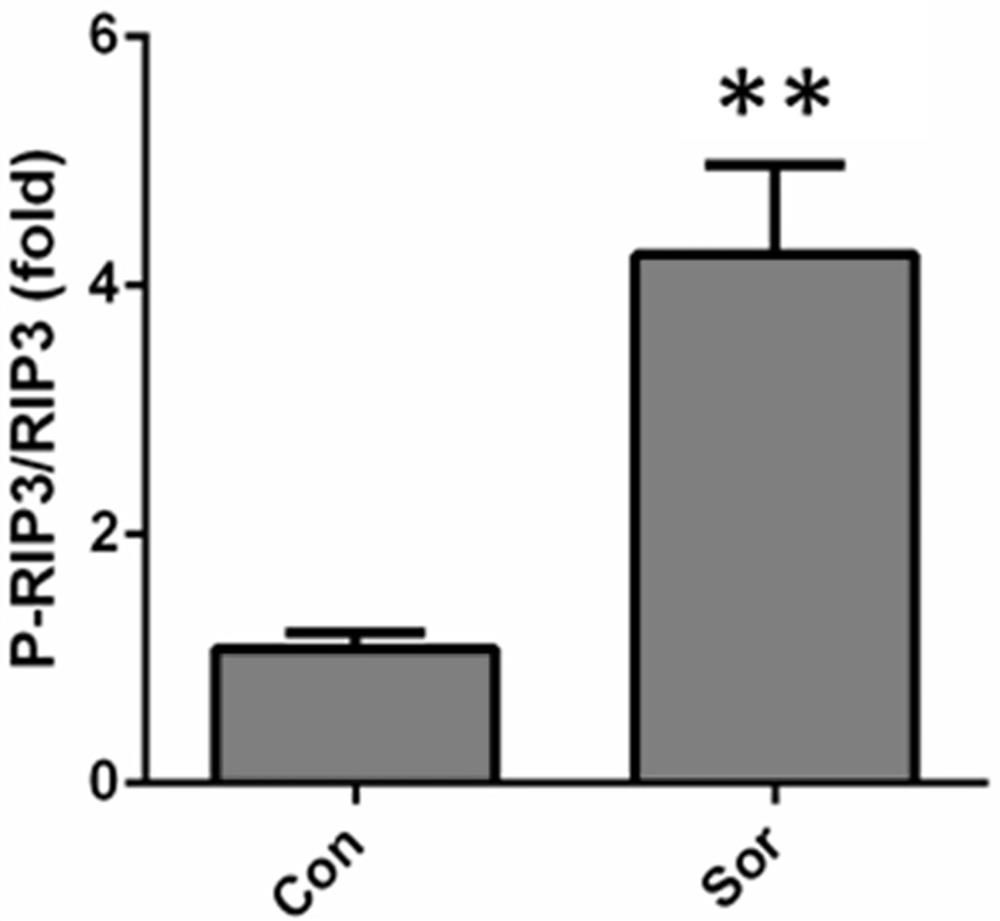
[0046] Example 2 Sarafini intervention induces cardiac tissue and cellular procedural death.
[0047] 1. Western blot Detects myocardial tissue procedural death protein expression.
[0048] Western blot method detection procedural death marker protein RIP3 and P-RIP3 expression. The protein concentration was measured by the RIPA lysate to extract the control group and the Solafini intervention group, and the protein concentration was determined using a BCA colorimetric kit. The expression of CREG protein was detected with Western Blot.
[0049] Specifically, the 40 μg of protein was boiled at 95 ° C for 5 min, and the hydraulic SDS-PAGE electrophoresis was separated by 10%, and the electrophoresis ended time. Samples were transferred to the cellulose membrane at 90V, and the time was 2 h; after normal temperature was closed for 2 hours in TBS-T dilution, it was added to incubate overnight at 4 ° C. The anti-RIP3 antibody (1: 1000, US ABCAM), anti-P-RIP3 antibody (1: 1000, US ABCAM...
Embodiment 3
[0058] Example 3 The expression of CREG1 in myocardial tissue in Sarafini decreased.
[0059] 1. Fluorescence Quantitative PCR Method Detects the expression of Creg1 gene mRNA in the front of the myocardial tissue before Sarafini.
[0060] The tissue RNA was extracted with a Promega kit and a TAKARA reverse transcription kit was used to obtain a reverse transcription reaction to obtain cDNA, followed by a quantitative PCR reaction with a Sybgreen method. Quantitative PCR primer sequences are as follows:
[0061]
[0062] The results showed that there was no significant change in the expression level of CREG gene mRNA in Sarafini group compared to the control group. Figure 2A Indicated.
[0063] 2, Western blot to detect the expression of CREG protein in myocardial tissue.
[0064] In order to detect the expression of CREG in myocardial tissue induced by Sarafini, CREG protein expression was detected by WesternBlot. Specific methods are shown in Example 1. The anti-CREG antibody ...
Embodiment 4
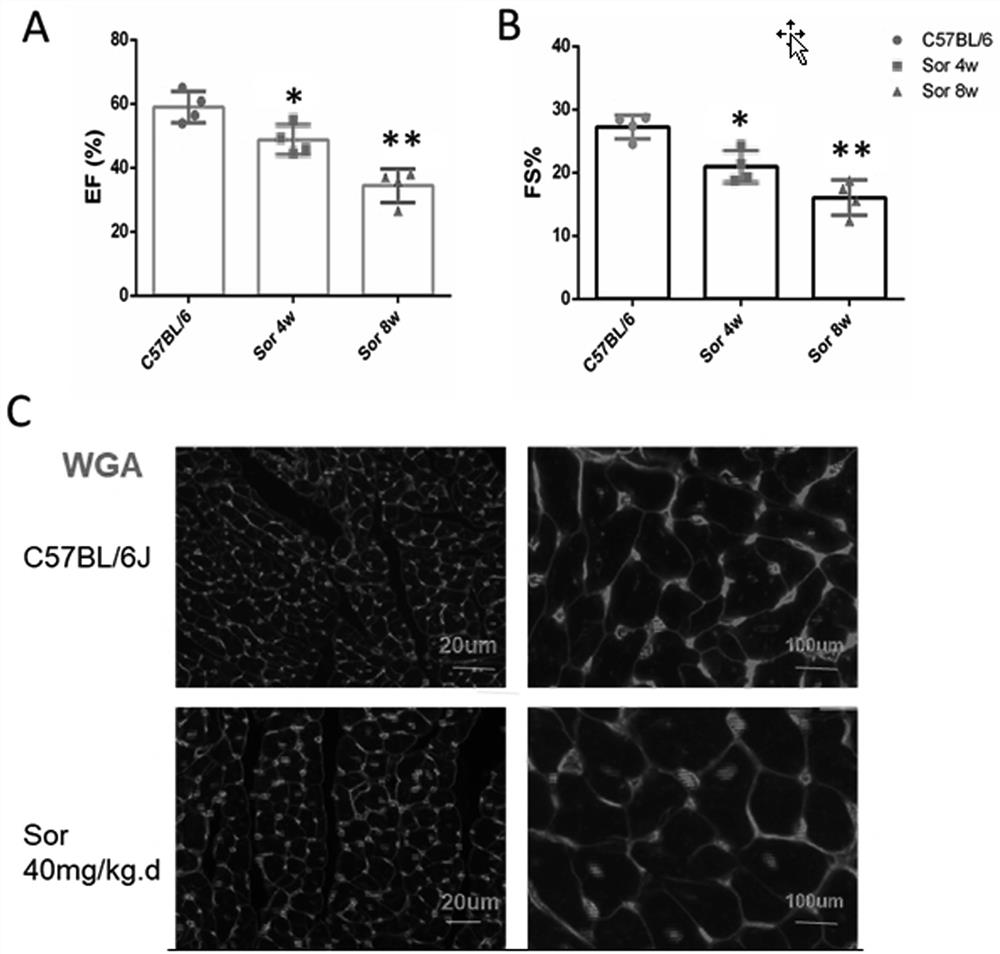
[0066]Example 4 Exogenous administration CREG recombinant protein significantly reduced C57BL / 6J mouse heart functional damage in Sarafini intervention.
[0067] 1. Exogenous CREG protein intervention is administered on the model of Sarafini-induced heart function injury.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com