Method of preparing diisocyanate composition and optical lens
A technology of diisocyanate and optical lens, applied in the field of preparing optical lens, can solve the problems of high toxicity of phosgene gas, increase of reactor pressure, and inability to smoothly perform stirring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
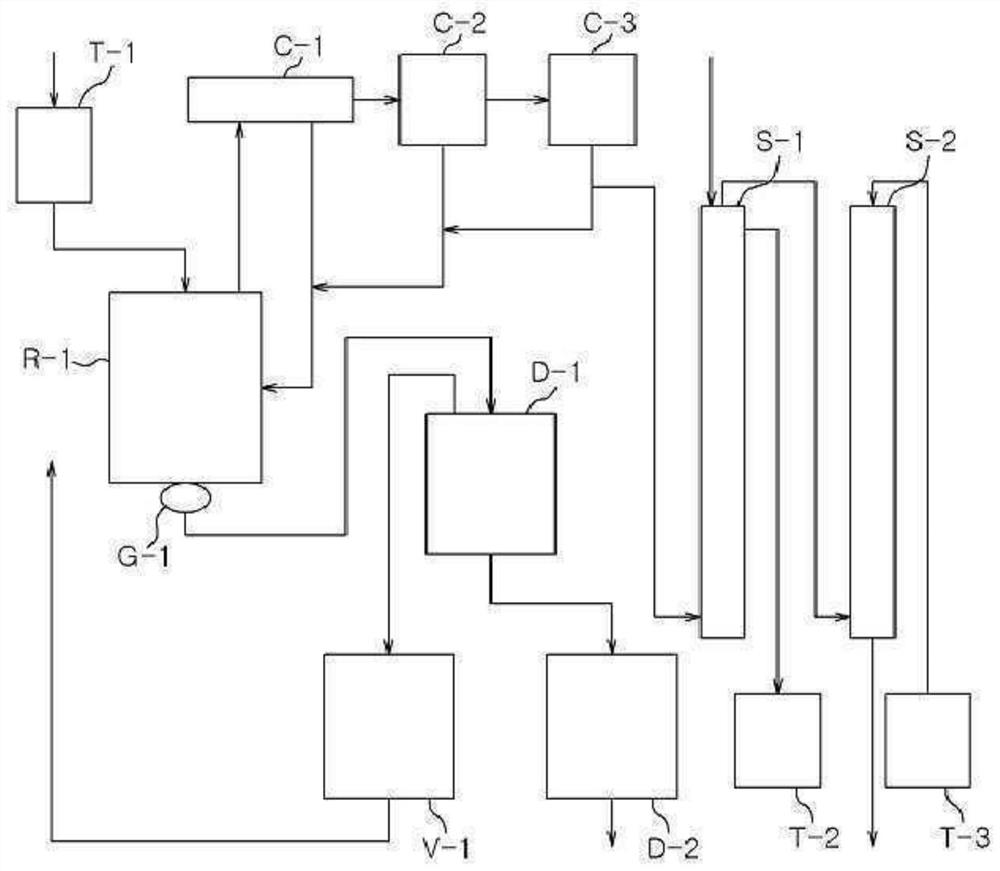
Image
Examples
example 5
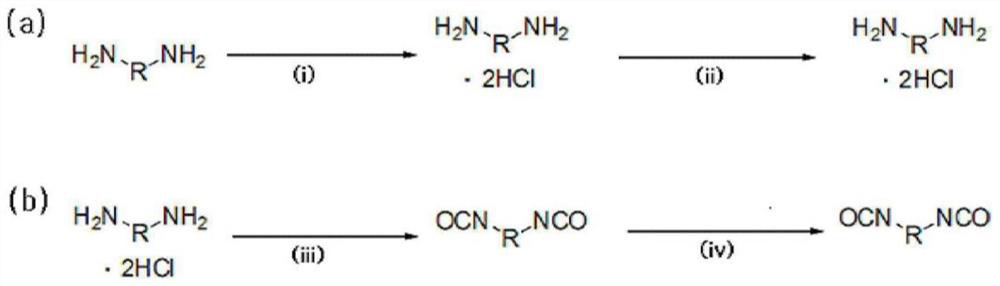
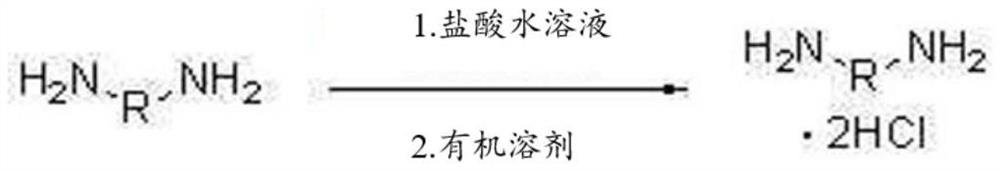
[0175] Step (1): Preparation of diamine hydrochloride composition
[0176] Reactor 1 was charged with 1,009.4 g (9.46 moles) of 35% aqueous hydrochloric acid (with the Fe ion content shown in Table 2 below), and then the internal temperature of Reactor 1 was lowered to 15° C. with stirring. While maintaining the temperature of Reactor 1 at 50° C. or lower, 627.0 g (4.4 moles) of H6XDA were introduced for 1 hour. After the introduction was completed, the internal temperature of the reactor was lowered to 10° C., and it was stirred for 1 hour. The internal temperature of Reactor 2 charged with 2,640.0 g of diethyl ether was lowered to -5°C. The mixture in Reactor 1 was slowly added dropwise to Reactor 2 at 0°C or lower.
[0177] After the addition was complete, the H6XDA·2HCl-containing diamine hydrochloride composition was separated by vacuum filtration using a filter and the filtered diethyl ether was recovered for reuse. Thereafter, the separated diamine hydrochloride comp...
example 6
[0181] Step (1): Preparation of diamine hydrochloride composition
[0182] 1,009.4 g (9.46 mol) of 35% aqueous hydrochloric acid solution (with Fe ion content shown in Table 2 below) was charged into the reactor, and then the internal temperature of the reactor was lowered to 15° C. with stirring. While maintaining the temperature of the reactor at 50° C. or lower, 490.1 g (4.4 moles) of HDA were introduced for 1 hour. After the introduction was completed, the internal temperature of the reactor was lowered to 10° C., and it was stirred for 1 hour. Thereafter, 1,320.0 g of tetrahydrofuran was introduced, and the internal temperature of the reactor was lowered to -5° C., followed by stirring for 1 hour. After the reaction, the diamine hydrochloride composition containing HDA·2HCl was separated by vacuum filtration using a filter, and the filtered tetrahydrofuran was recovered for reuse. Thereafter, the separated diamine hydrochloride composition was vacuum-dried at 90° C. and...
example 7
[0186] Step (1): Preparation of diamine hydrochloride composition
[0187] 1,009.4 g (9.46 moles) of 35% aqueous hydrochloric acid (with Fe ion content shown in Table 2 below) was charged into Reactor 1, and then the internal temperature of Reactor 1 was lowered to 15° C. with stirring. While maintaining the temperature of Reactor 1 at 50° C. or lower, 812.0 g (4.4 moles) of IPDA were introduced for 1 hour. After the introduction was completed, the internal temperature of the reactor was lowered to 10° C., and it was stirred for 1 hour. The internal temperature of Reactor 2 charged with 2,640.0 g of diethyl ether was lowered to -5°C. The mixture in Reactor 1 was slowly added dropwise to Reactor 2 at 0°C or lower. After the addition was complete, the IPDA·2HCl-containing diamine hydrochloride composition was separated by vacuum filtration using a filter and the filtered diethyl ether was recovered for reuse. Thereafter, the separated diamine hydrochloride composition was vac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| haze | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com