A porous ion-conducting membrane for a flow battery and its preparation and application
A technology for ion-conducting membranes and flow batteries, which is applied in the field of porous ion-conducting membranes for flow batteries and its preparation and application. It can solve the problems of low conductivity and poor selectivity of all-vanadium redox flow battery membranes, and achieve high ion conductivity. efficiency, excellent battery performance, and high ion selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
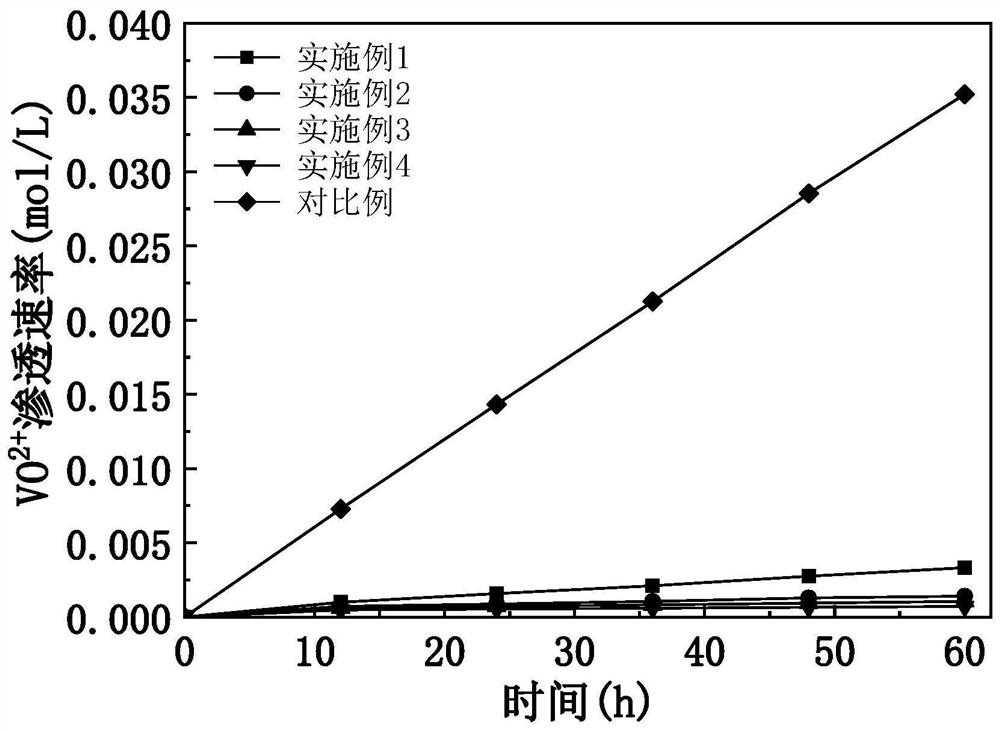
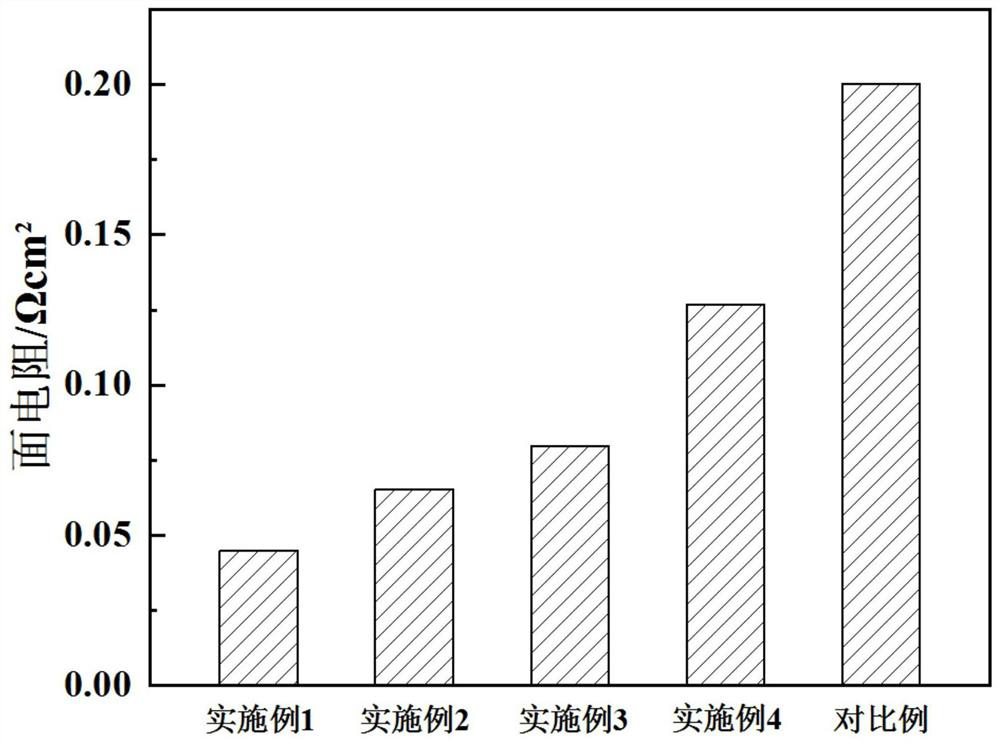
Examples
Embodiment 1
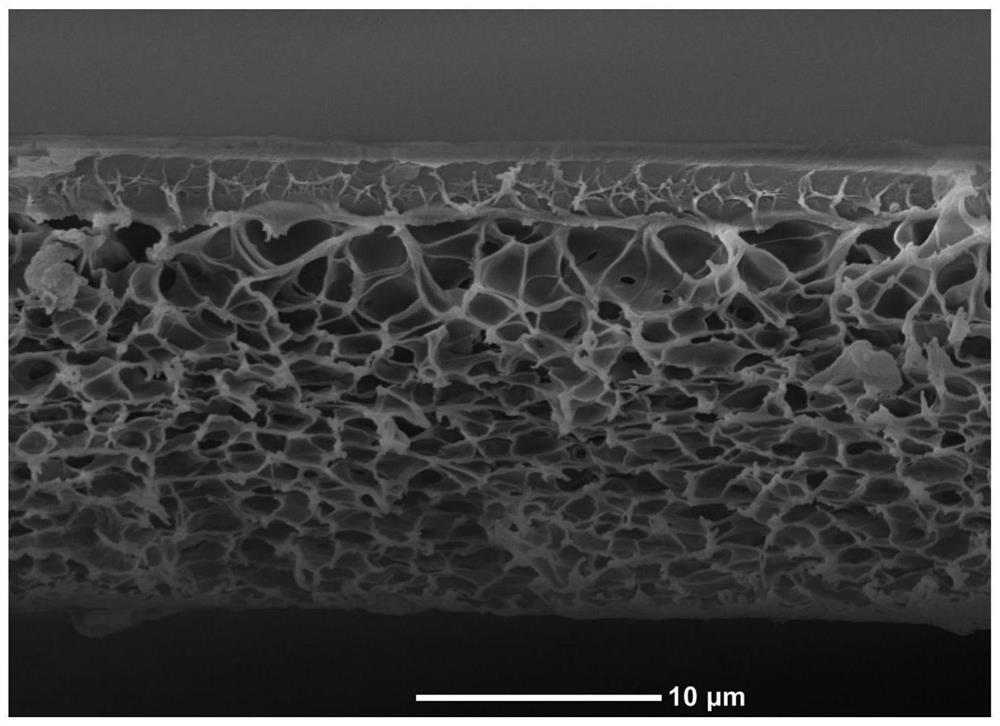
[0024] 6 g of polybenzimidazole (PBI) was dissolved in 44 g of DMAc and stirred at room temperature for 48 hours to form a polymer solution. Prepare a homogeneous solution of 140g n-heptane and 35g 1,2-dibromoethane, spread the polymer solution on a glass plate, volatilize the solvent for 5s, then immerse the glass plate in n-heptane and 1,2 -Dibromoethane mixed solution for 10s, then transferred to a tank filled with deionized water at 25°C and completely submerged until solidified to form a film to obtain ions with a dense separation layer and a loose porous support layer (pore size distribution 100-200nm) Conductive membrane, the thickness of the separation layer is 1±0.5um, the porosity of the membrane is about 75%, and the thickness of the membrane is 18±2μm. Soak in 3mol L-1 sulfuric acid solution before use.
Embodiment 2
[0026] 6 g of polybenzimidazole (PBI) was dissolved in 44 g of DMAc and stirred at room temperature for 48 hours to form a polymer solution. Prepare a homogeneous solution of 81.67g n-hexane and 35g 1,2-dibromoethane, spread the polymer solution on a glass plate, evaporate the solvent for 5s, then immerse the glass plate in n-heptane and 1,2 - Dibromoethane mixed solution for 20s, then transferred to a tank filled with 25 ° C deionized water and completely submerged until solidified into a film, to obtain ions with a dense separation layer and a loose porous support layer (pore size distribution 100-200nm) Conductive membrane, the thickness of the separation layer is 3±0.5um, the porosity of the membrane is about 71%, and the thickness of the membrane is 18±2μm. Soak in 3mol L-1 sulfuric acid solution before use.
Embodiment 3
[0028] 7.5g of polybenzimidazole (PBI) was dissolved in 42.5g of DMAc and stirred at room temperature for 48 hours to form a polymer solution. Prepare a homogeneous solution of 65g n-heptane and 35g chloroform, spread the polymer solution on a glass plate, volatilize the solvent for 5s, and then immerse the glass plate in n-heptane and 1,2-dibromoethane Mixed solution for 20s, then transferred to a water tank filled with 25°C absolute ethanol and completely submerged until solidified to form a film to obtain an ion-conducting membrane with a dense separation layer and a loose porous support layer (pore size distribution 100-200nm), the separation layer The thickness is 3±0.5um, the membrane porosity is about 70%, and the membrane thickness is 18±2μm. Soak in 3mol L-1 sulfuric acid solution before use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com