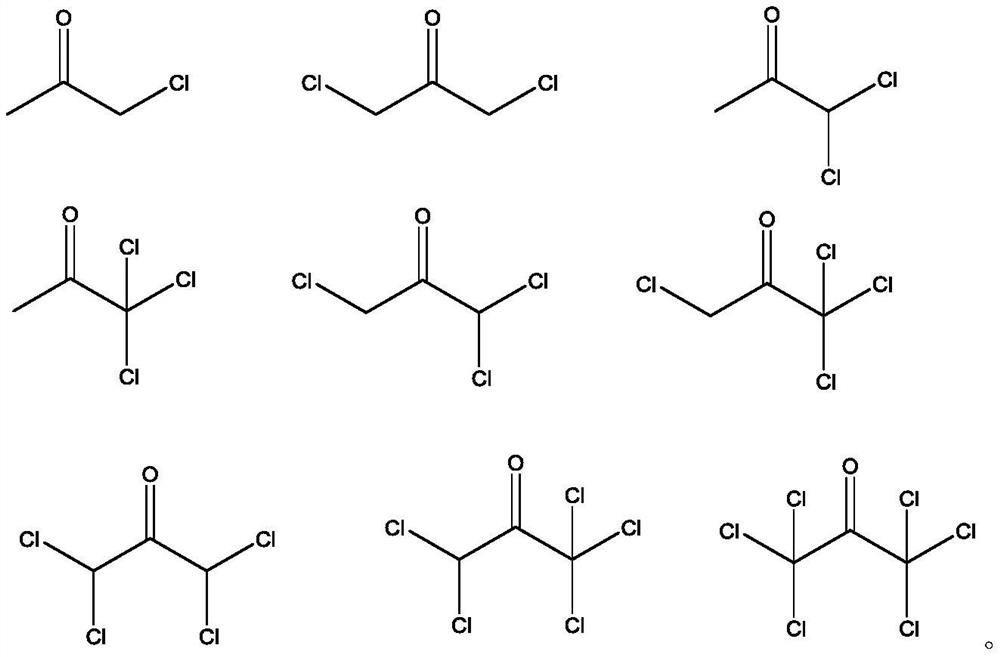
Method for detecting chloroacetone compounds in folic acid
A detection method, the technology of chloroacetone, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem of low accuracy of detection results, and achieve the effects of accurately determining chloroacetone compounds, improving solubility, and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Solution preparation.
[0041] Same method as in method verification step.
[0042] (2) Chromatographic detection.
[0043] Using a Thermo TRACE 1300 chromatograph, test the blank solution, the test solution and the reference solution in sequence, and the test working conditions are as follows:
[0044] Chromatographic column: Agilent DB-624, with a length of 30m, a diameter of 0.32mm, a film thickness of 1.8μm, and an operating temperature range of -20°C to 260°C.
[0045] Injection volume: 1 μL, inlet temperature: 200°C, split ratio 5:1, column temperature 70°C, temperature was raised to 180°C at 20°C / min, and then to 240°C at 10°C / min.
[0046] Carrier gas: high-purity nitrogen; hydrogen flow: 35mL / min; air flow: 350mL / min; make-up gas: 40mL / min
[0047] Detector: FID; Detector temperature: 250°C.
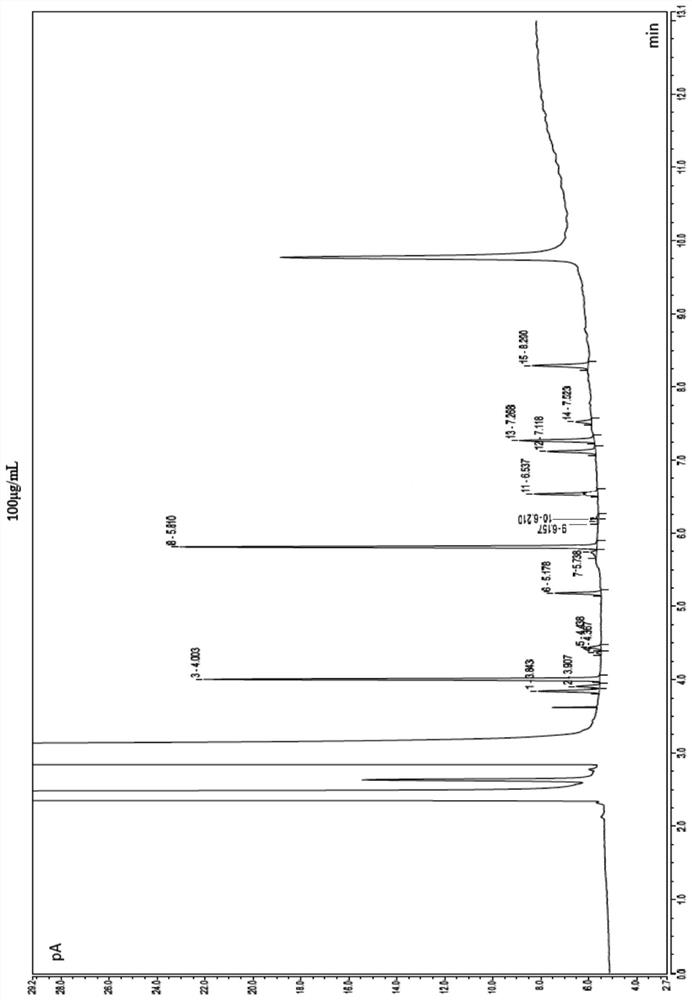
[0048] see test results figure 1 , monochloroacetone peaks at position 3 with a retention time of 4.003 min; 1,1-dichloroacetone peaks at position 5 with a reten...
Embodiment 2
[0050] (1) Solution preparation.
[0051] Same method as in method verification step.
[0052] (2) Chromatographic detection.
[0053] Using a Thermo TRACE 1300 chromatograph, test the blank solution, the test solution and the reference solution in sequence, and the test working conditions are as follows:
[0054] Chromatographic column: Agilent DB-624, with a length of 30m, a diameter of 0.32mm, a film thickness of 1.8μm, and an operating temperature range of -20°C to 260°C.
[0055] Injection volume: 1 μL, inlet temperature: 200°C, split ratio 5:1, column temperature 70°C, temperature was raised to 180°C at 20°C / min, and then to 240°C at 10°C / min.
[0056] Carrier gas: high-purity nitrogen; hydrogen flow: 35mL / min; air flow: 350mL / min; make-up gas: 40mL / min
[0057] Detector: ECD; Detector temperature: 250°C.
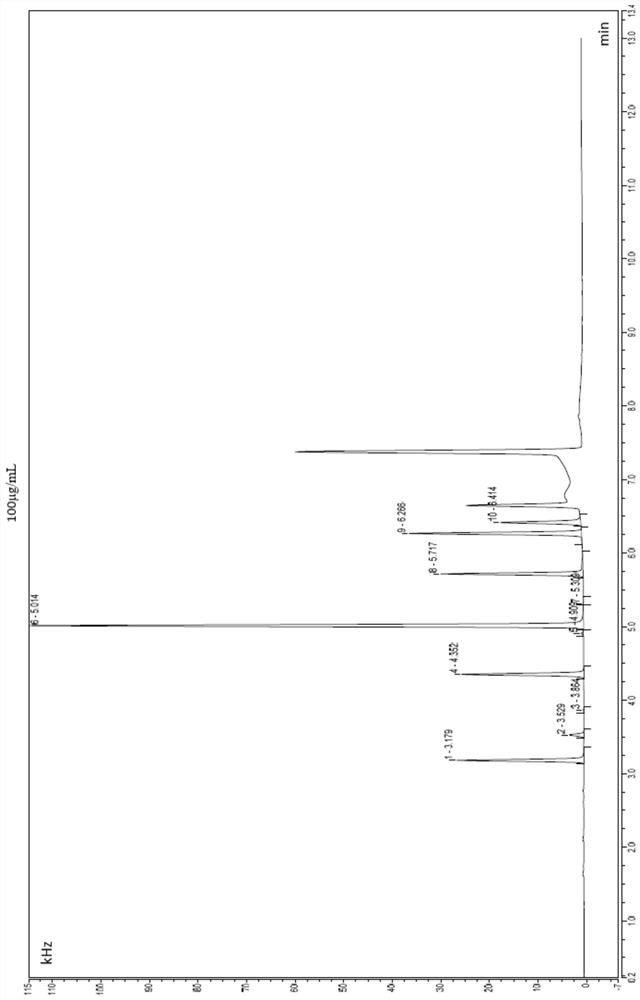
[0058] see test results figure 2 , when the test sample is 100μg / ml, monochloroacetone peaks at position 1 with a retention time of 3.179min; 1,1-dichloroaceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com